
- A researcher from the University of Granada has participated in an international study that successfully identified—from a virtual library of over 300,000 materials classified as metal–organic frameworks—an optimal material for capturing CO2. The work was recently published in the prestigious journal Nature
Climate change appears to be related to the anthropogenic emission of carbon dioxide (CO2) generated by intensive use of fossil fuels. The development of efficient technologies for CO2 capture and storage has been found to be the most viable approach to mitigating this problem.
Professor Jorge Rodríguez Navarro, a researcher at the Department of Inorganic Chemistry of the University of Granada (UGR), has participated in an international study published in the journal Nature in which ‘big data’ techniques were used to select an optimal material for CO2 capture from a virtual library of more than 300,000 materials classified as metal–organic frameworks (MOFs).
The results showed that the materials analysed outperformed classical porous materials, such as zeolite and activated carbon, when subjected to typical conditions of CO2 capture in thermal power plants.
Drug-design and screening methodology
The methodology used in the trial was similar to that used in the selection of drugs by the pharmaceutical industry, in which a drug that fits well into the binding pocket of a protein that causes a given disease is mined from databases of known molecules.
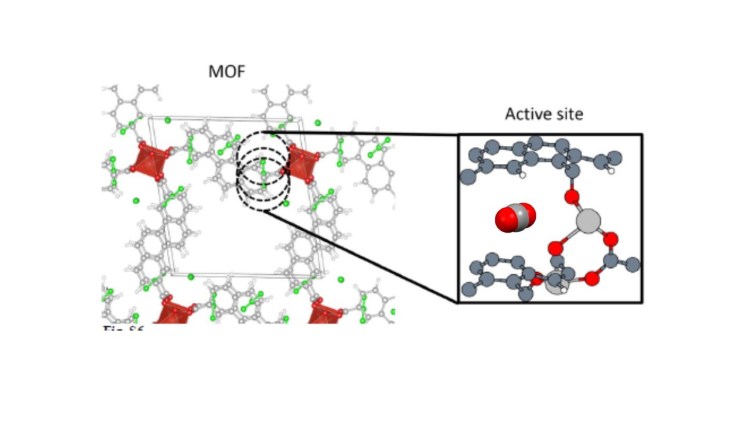
In this case, the target molecule was known (CO2), while the optimal material was not. “Using this ‘big data’ technique enabled us to identify the binding pocket presented by the best-performing materials, for which we coined the term ‘adsorbaphore’”, comments the author.
This ‘adsorbaphore’ of the CO2 molecule consisted of two aromatic rings spaced 7 angstroms apart, which were capable of selectively binding a CO2 molecule, rather like a molecular ‘sandwich’ (see figure).
Once the theoretically-optimal materials had been selected, they were synthesized and their performance studied in terms of CO2 capture.
Bibliography:
Boyd, P.G., Chidambaram, A., García-Díez, E. et al. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature 576, 253–56 (2019). doi:10.1038/s41586-019-1798-7
www.nature.com/articles/s41586-019-1798-7
Image captions:

Media enquiries:
Jorge A. Rodriguez Navarro, Department of Inorganic Chemistry, University of Granada
Tel.: + 34 958 248093
Email: @email
www.ugr.es/local/jarn



