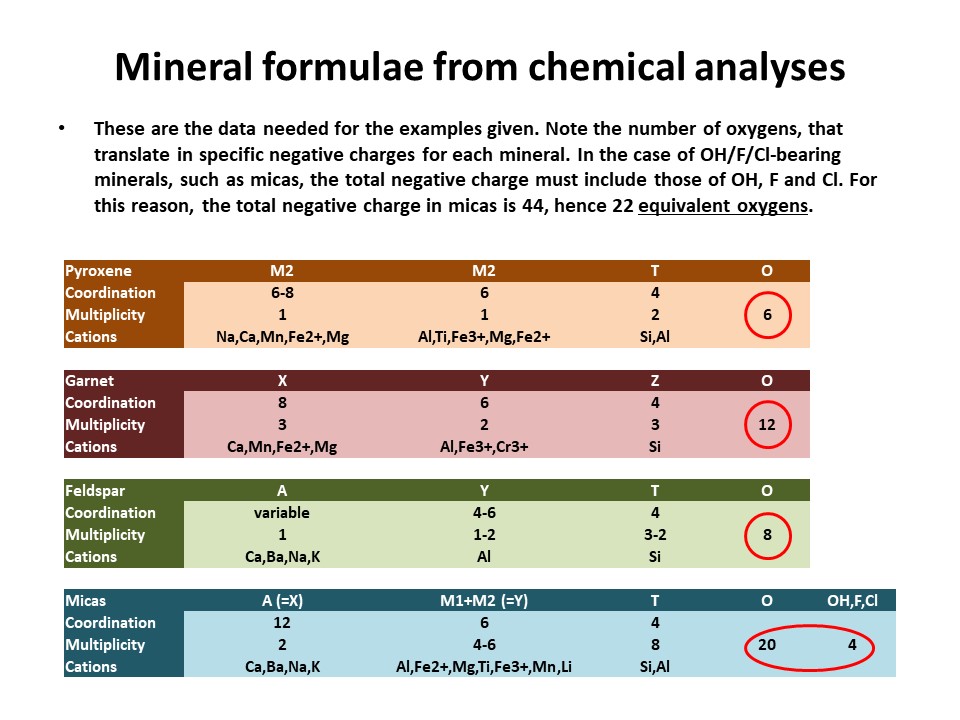
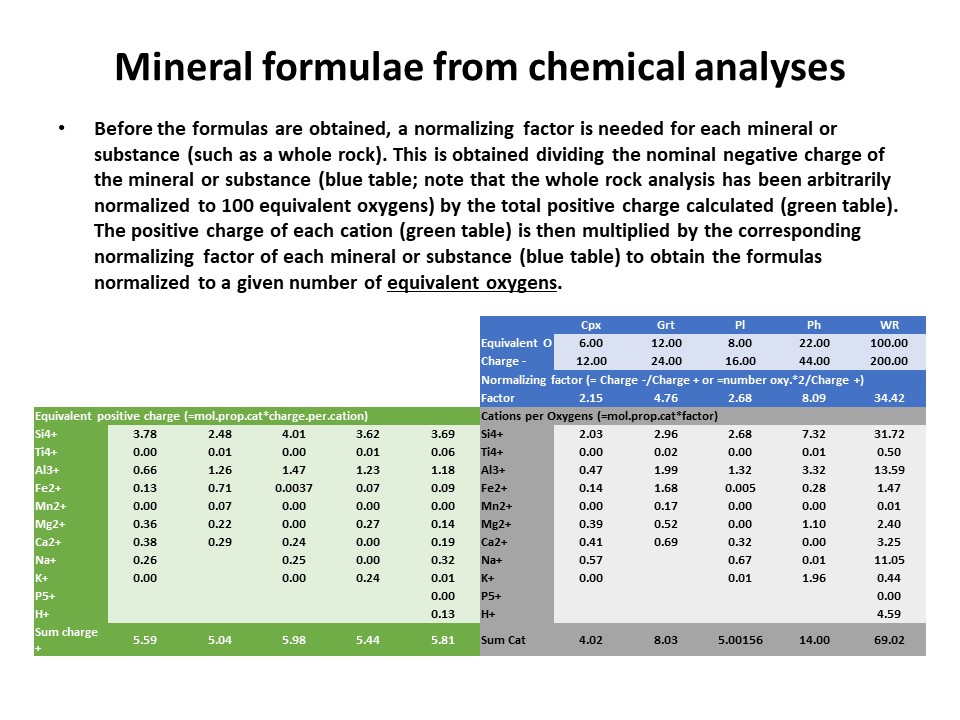
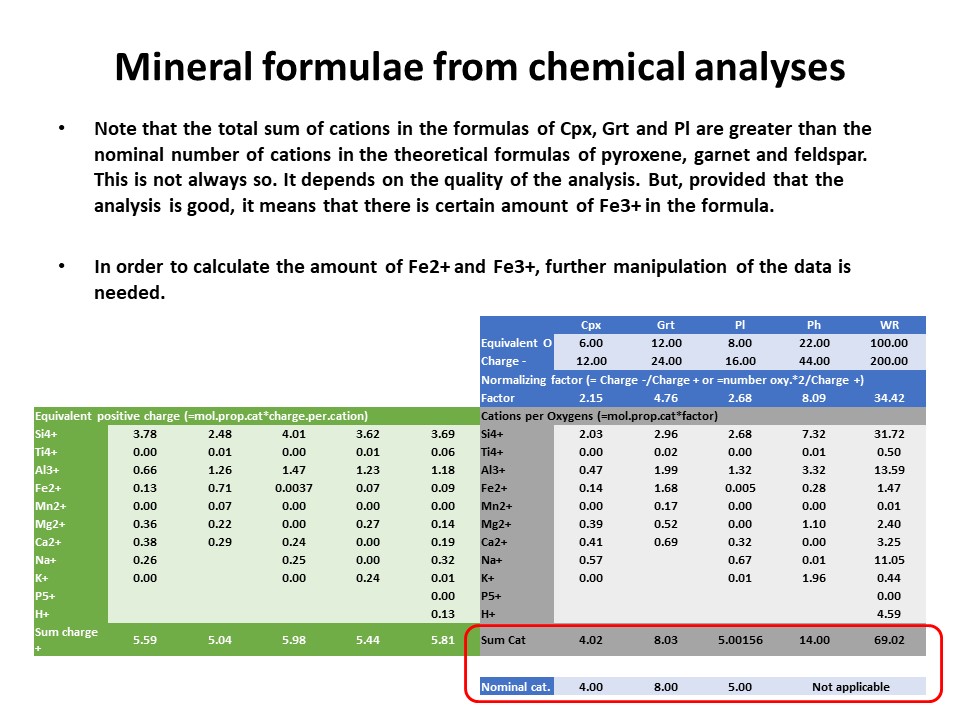
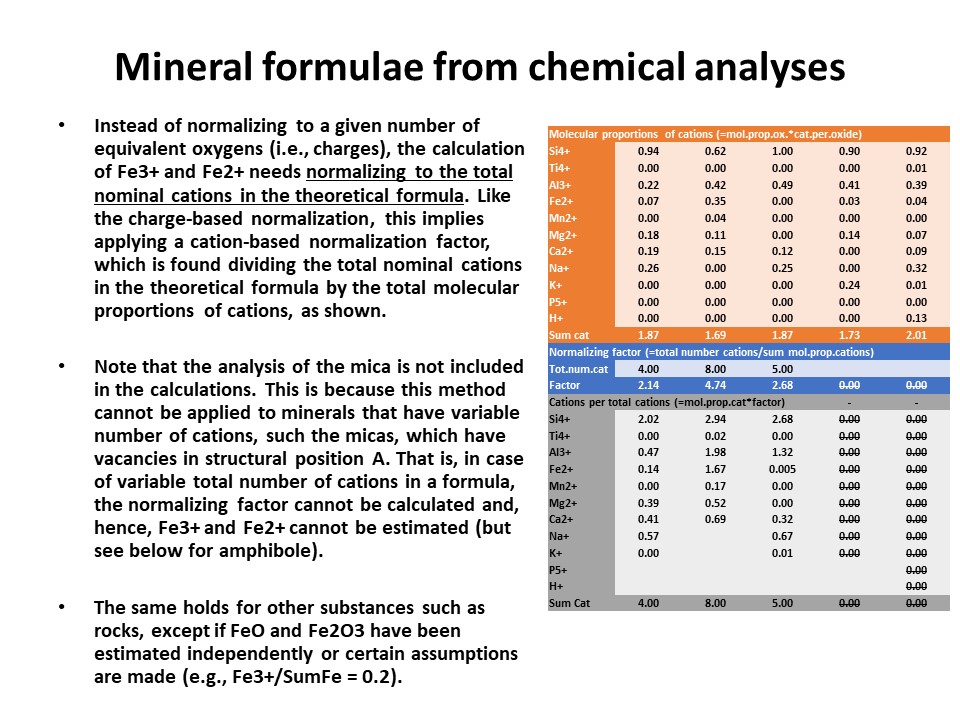
|


Suggested further reading


Greenwood, 1975:
http://www.minsocam.org/ammin/AM60/AM60_1.pdf
Greenwood, 1967:
https://doi.org/10.1016/0016-7037(67)90029-4
Spear
et al., 1982:
https://www.degruyter.com/document/doi/10.1515/9781501508172-007/html,
or
Spearetal1982
Fisher, 1989:
https://doi.org/10.1007/BF01160191
Fisher, 1993:
http://www.minsocam.org/ammin/AM78/AM78_1257.pdf

CSpace website:
https://www.ugr.es/local/agcasco/cspace/
CSpace website:
http://www.ugr.es/local/agcasco/personal/ >> CSpace
Torres-Roldan et al., 2000:
https://doi.org/10.1016/S0098-3004(00)00006-6
See also:
https://serc.carleton.edu/research_education/equilibria/index.html
https://serc.carleton.edu/research_education/equilibria/mineralformulaerecalculation.html
https://serc.carleton.edu/research_education/equilibria/phaserule.html
https://serc.carleton.edu/research_education/equilibria/simplephasediagrams.html
https://serc.carleton.edu/research_education/equilibria/metamorphic_diagrams.html
https://serc.carleton.edu/research_education/equilibria/pseudosections.html
https://serc.carleton.edu/research_education/equilibria/chem_projections.html
https://serc.carleton.edu/research_education/equilibria/balancingmetareactions.html
https://serc.carleton.edu/research_education/equilibria/schreinemakers.html
"The student.... is urged... to concentrate on forming a mental
image of the appearance of the composition space". Frank Spear,
1995.

See the crystal structure of almandine garnet
(Fe3Al2Si3O12) in MINDAT database:
https://www.mindat.org/min-452.html
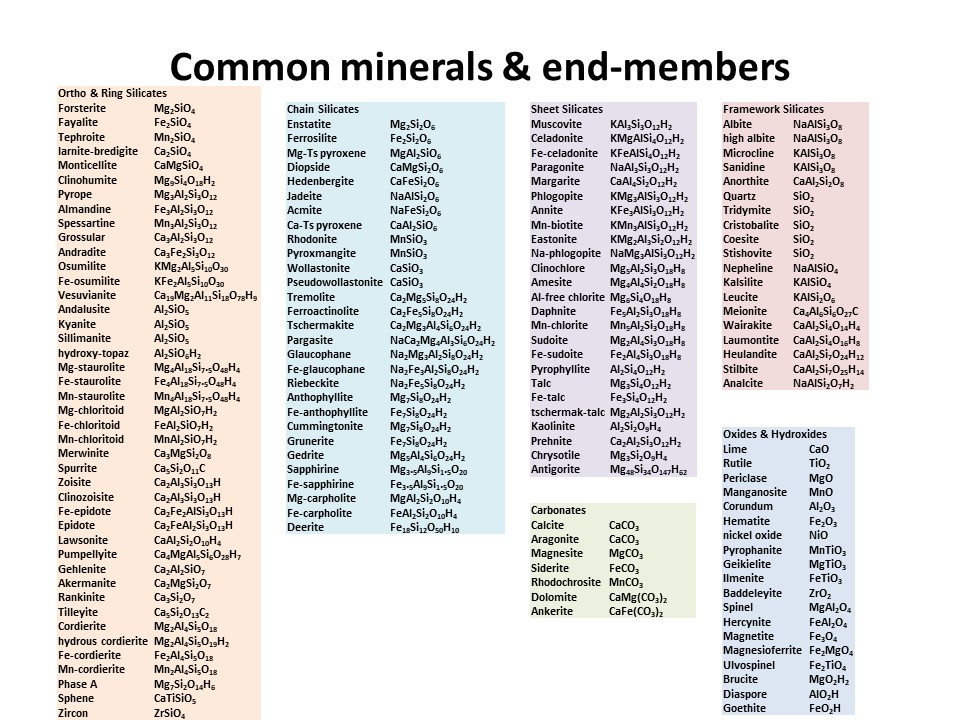
Basic crystalchemistry of common minerals (in Spanish):
https://www.ugr.es/~agcasco/personal/Cristalquimica/Cristalquimica.htm
MINDAT: https://www.mindat.org/
WEBMINERAL:
http://www.webmineral.com/
Handbook of Mineralogy:
http://www.handbookofmineralogy.org/index.html
IMA Reports published in the American Mineralogist
Nomenclature of the garnet supergroup, 2013
Edward S. Grew et al. pdf
(2.3 MB)
Nomenclature of the amphibole supergroup, 2012
Frank C. Hawthorne et al. pdf
(4.6 MB)
Named Amphiboles: A new category of amphiboles recognized by the
International Mineralogical Association (IMA) and a defined sequence order for
the use of prefixes in amphibole names, 2005
Ernst A.J. Burke And Bernard E. Leake pdf
(84 KB)
Nomenclature of amphiboles: Additions and revisions to the International
Mineralogical Association's amphibole nomenclature, 2004
Bernard E. Leake et al. pdf
(188 KB)
IMA Reports prior to 1998 published in the Canadian Mineralogist
Robert F. Martin, editor The Canadian Mineralogist, has kindly let the
Mineralogical Society of American host a set of International Mineralogical
Association Commission on New Minerals and Mineral Names reports that were
compiled by the Mineralogical Association of Canada and the Canadian
Mineralogist on the occasion of the IMA 17th General Meeting in Toronto (August
1998). These reports are also available at the Mineralogical
Association of Canada both as an electronic
version and as a Booklet.
On the use of names, prefixes and suffixes, and adjectival modifiers in the
mineralogical nomenclature, 1980
M.H. Hey and G. Gottardi pdf
(176 KB)
The definition of a mineral, 1995
E.H. Nickel pdf
(264 KB)
Formal definitions of type mineral specimens, 1987
P.J. Dunn and J.A. Mandarino pdf
(236 KB)
Solid solutions in mineral nomenclature, 1992
E.H. Nickel pdf
(324 KB)
Nomenclature of the micas, 1998
M. Rieder et al. pdf
(412 KB)
Nomenclature of amphiboles: report of the Subcommittee on Amphiboles of the
International Mineralogical Association, Commission on New Minerals and Mineral
Names, 1997
B.E. Leake et al. pdf
(1.2 MB)
Nomenclature of pyroxenes, 1989
N. Morimoto et al. pdf
(1.5 MB)
Recommended nomenclature for zeolite minerals: report of the Subcommittee on
Zeolites of the International Mineralogical Association, Commission on New
Minerals and Mineral Names, 1997
D.S. Coombs et al. pdf
(340 KB)
Classification and nomenclature of the pyrochlore group, 1977
D.D. Hogarth pdf
(1.0 MB)
Nomenclature of platinum-group-element alloys: review and revision, 1991
D.C. Harris and L.J. Cabr /i pdf
(1.1 MB)
Appendix. Symbols of the rock-forming minerals
After Kretz and Spear pdf
(140 KB)
Mineral abbreviations:
IMA (pdf).
Kretz (1983, Am
Min; html edited by A. Garcia-Casco)
Donna L. Whitney y Bernard W. Evans (2010; html
edited by A. Garcia-Casco)
Also:
American Mineralogist
Crystal Structure Database
IMA Mineral List
with Database of Mineral Properties
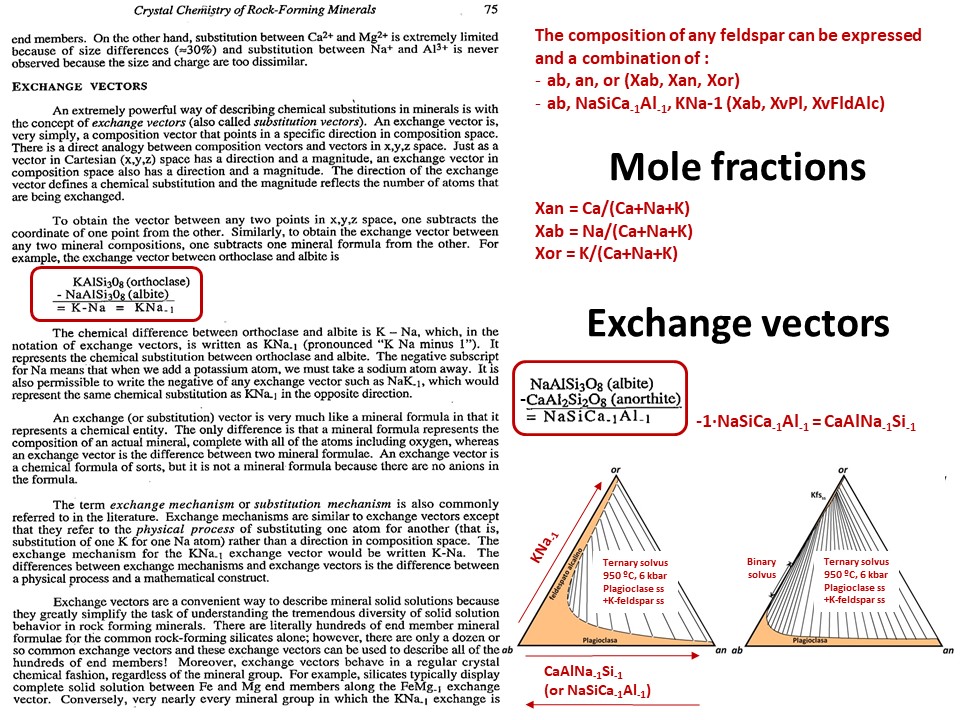
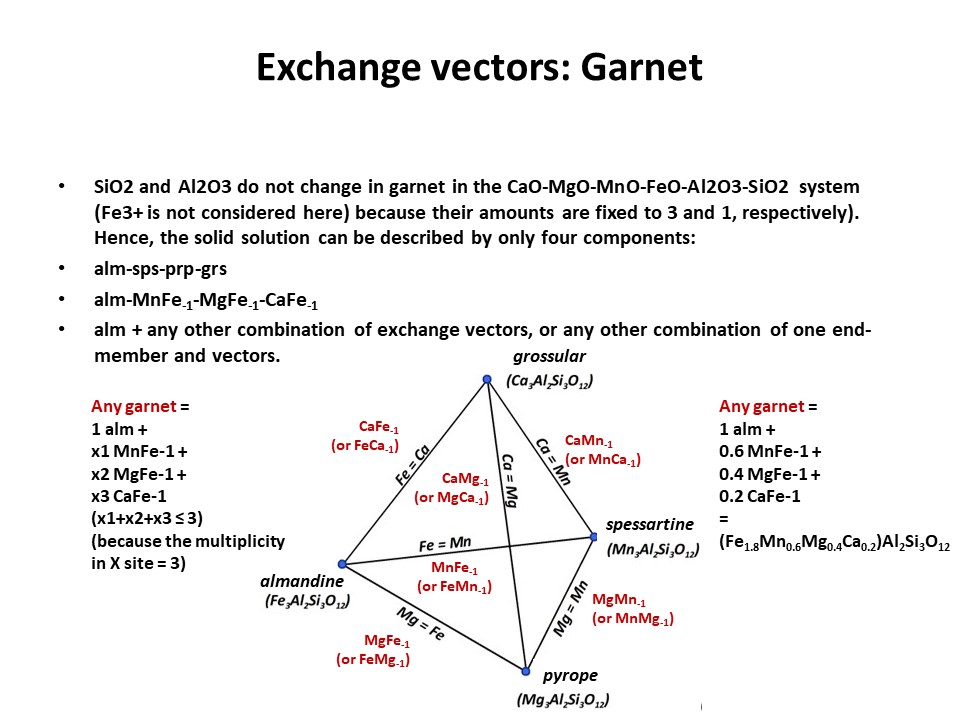
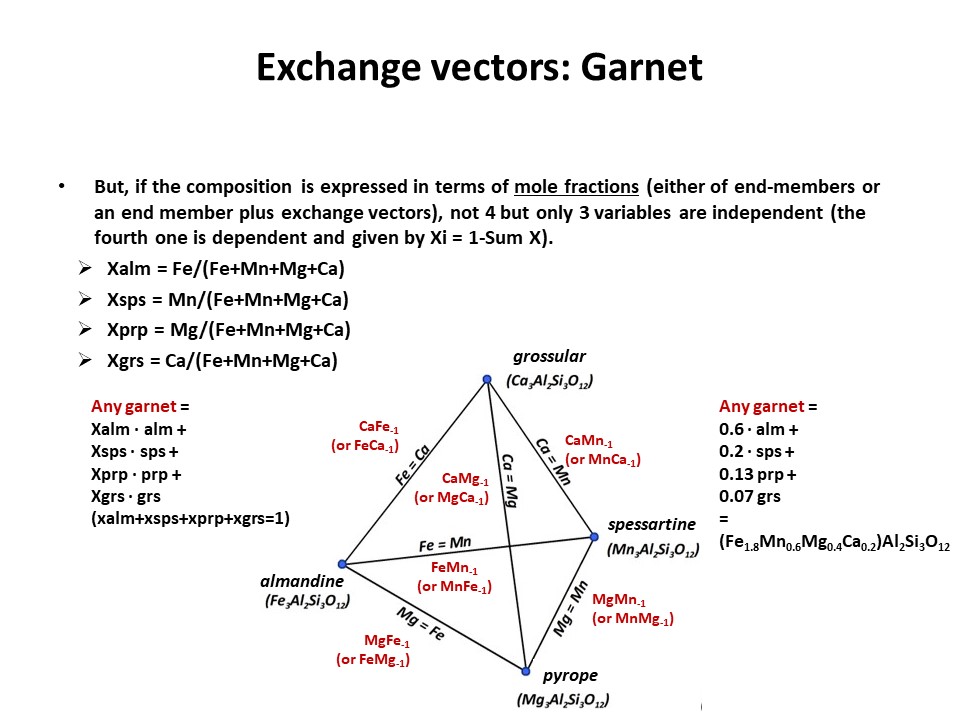
Solid solutions,
end-members
and exchange vectors

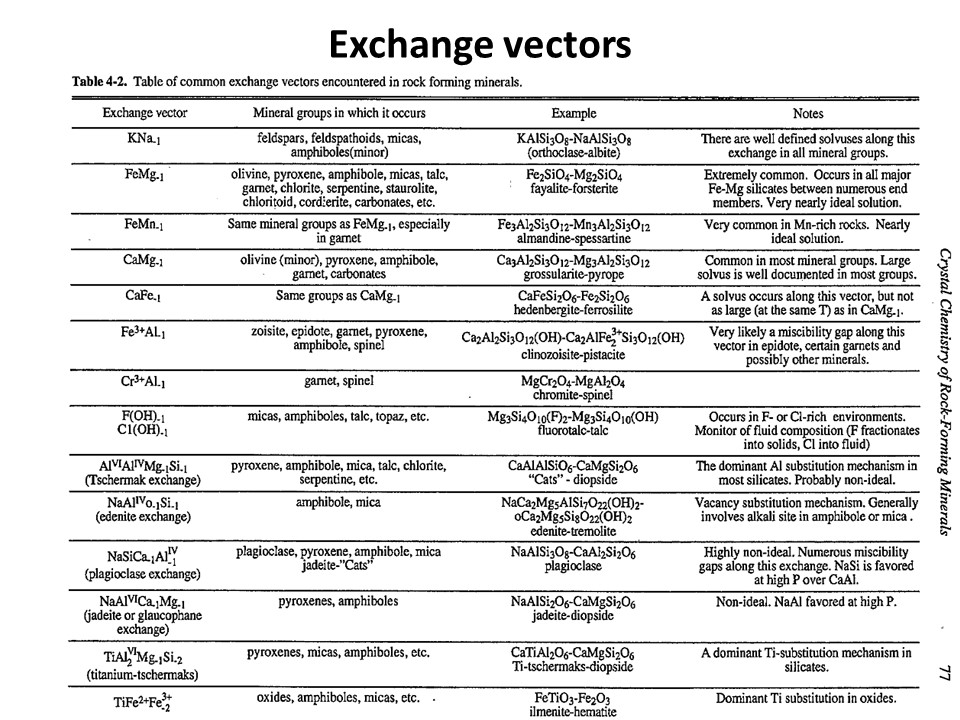
Exchange vectors: Mathematical operators (vectors)
that allow describing the changes in chemical composition of a phase.
They can be simple (e.g. KNa-1,
MgFe-1) o coupled (CaAlNa-1Si-1,
IVAlVIAlMg-1Si-1), and
involve cations, anions (Cl(OH)-1) and
vacant sites (VIMg3VIAl-2VI(o)-1,
ANaIVAlA(o)-1IVSi-1).
They may hence not maintain a mass-balance (equal number of
cations+anions in both sides of the vector, but they must
maintaing electrostatic balance (equal number of charges in both
sides of the vector). The exchange vectors normally work in more
than one phase (e.g., micas,
amphiboles, pyroxenes, chlorite, etc. etc.) and describe large
groups of phases, in particular solid solutions with limited
(partial) solution between them (e.g., dioctaedral and trioctaedral
micas: VIMg3VIAl-2VI(o)-1).
They can be applied to any phase, including solids, liquids, gasses
and fluids. When applied to solid solutions, the exchange vectors
inform on their cristalchemical behavior because they contain
structural information (for example, their formulation distinguishes
IVAl from VIAl or ANa from BNa).
The formulations of exchange vectors are obtained subtracting two
chemical species that represent end-members (or members, in general)
of the solid solutions. This is shown below for the feldspars.
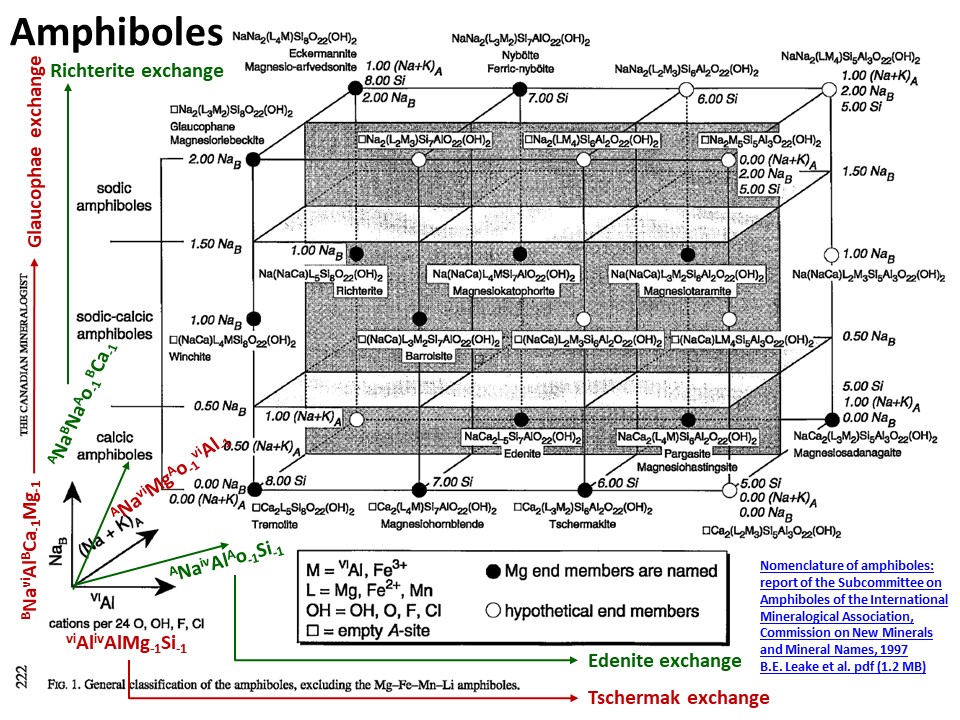
From: Bernard E. Leake; Alan R. Woolley; Charles E. S.
Arps; William D. Birch; M. Charles Gilbert; Joel D. Grice; Frank C.
Hawthorne; Akira Kato; Hanan J. Kisch; Vladimir G. Krivovichev; Kees
Linthout; Jo Laird; Joseph A. Mandarino; Walter V. Maresch; Ernest
H. Nickel; Nicholas M. S. Rock; John C. Schumacher; David C. Smith;
Nick C. N. Stephenson; Luciano Ungaretti; Eric J. W. Whittaker; Guo
Youzhi. Nomenclature of amphiboles; report of the subcommittee on
amphiboles of the International Mineralogical Association,
Commission on New Minerals and Mineral Names. The Canadian
Mineralogist (1997) 35 (1): 219–246. (https://pubs.geoscienceworld.org/canmin/article-lookup/35/1/219
or
http://www.minsocam.org/MSA/IMA/ima98(11).pdf).
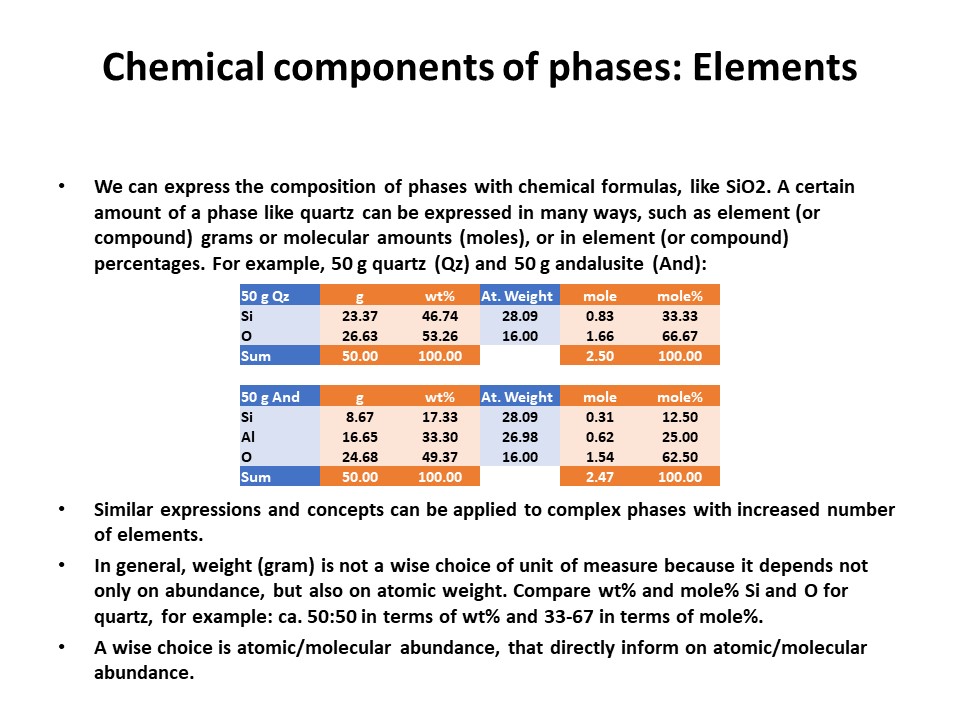
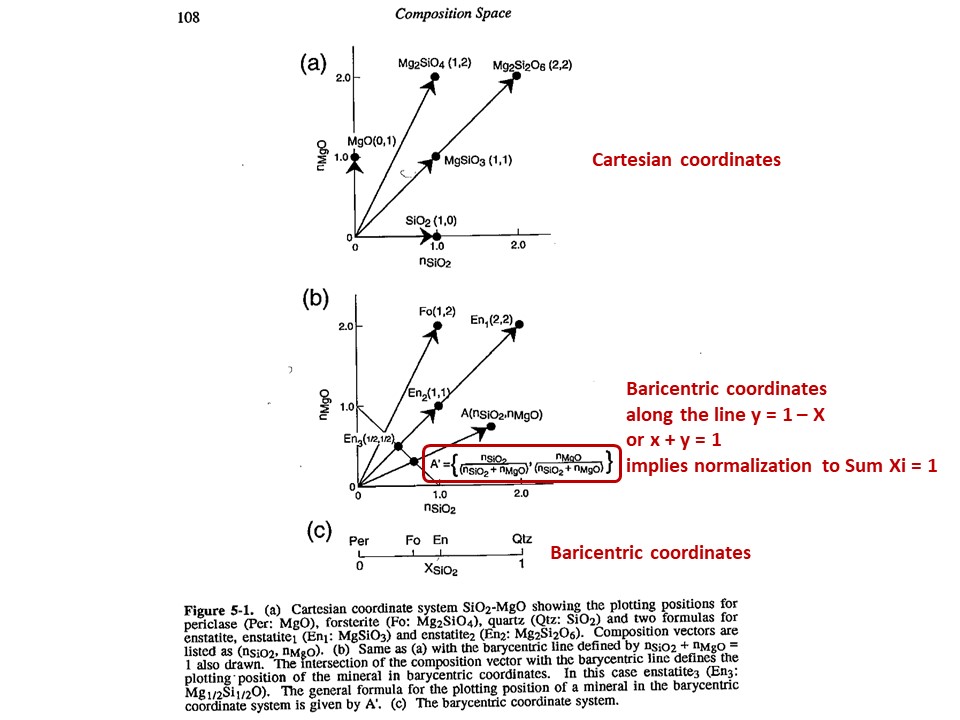
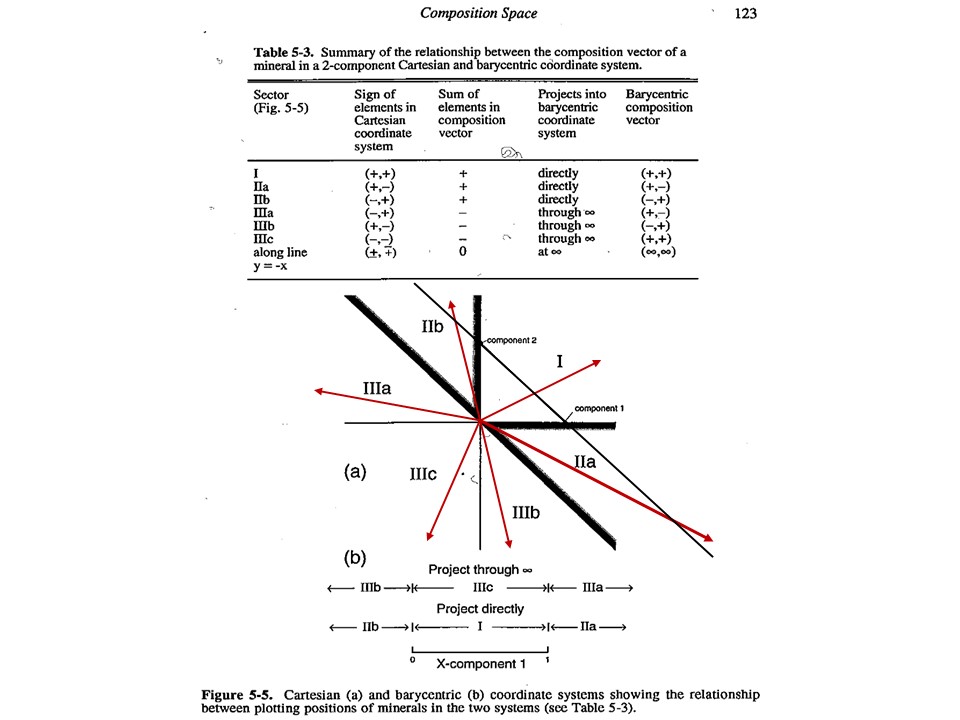
Composition
space
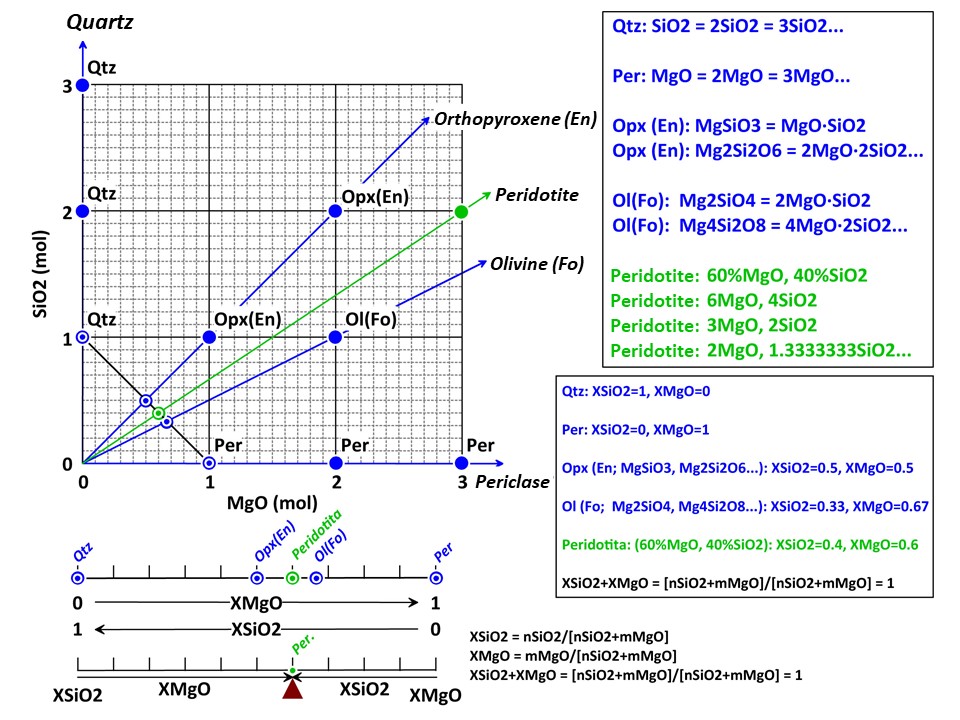
A simple chemical system with 2 dimensions: SiO2-MgO.
Relations between cartesian and baricentric projections. The
baricentric projection of a mineral vector (o the vector
corresponding to any chemical species that can be fully described by
the system) is found normalizing to 1:
XSiO2 = nSiO2/[nSiO2+nMgO]
XMgO = nMgO/[nSiO2+nMgO],
where nSiO2 and nMgO correspond to the cartesian molar values of
SiO2 and MgO and XSiO2 and XMgO are termed mole fractions. In
this normalization Sum Xi = 1.
The mole fractions, or the baricentric molar values of SiO2 and MgO,
represent the intersection of two lines in the cartesian space. One
line describes the mineral vector (y = a·x), while the other one
describes the locus of the baricentric projection, which is defined
as y = 1-x, or y+x = 1.
The same rules apply to any chemical system with n dimensions. Due
to normalization, 2 independent cartesian variables transform into 1
independent baricentric variable, hence allowing the baricentric
representation along a 1D line; 3 = 2D plane; 4 = 3D volume; 5,
6,... hyperplanes that cannot be fully represented, but can be
represented after reducing the dimension of the baricentric space
after condensation and projection (this is treated below).
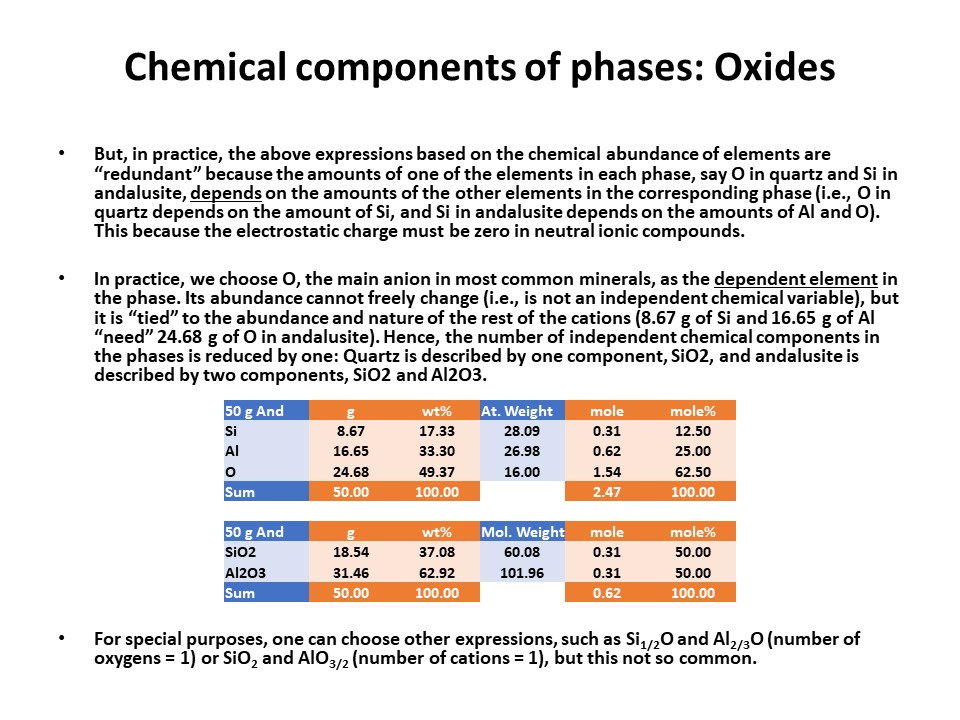
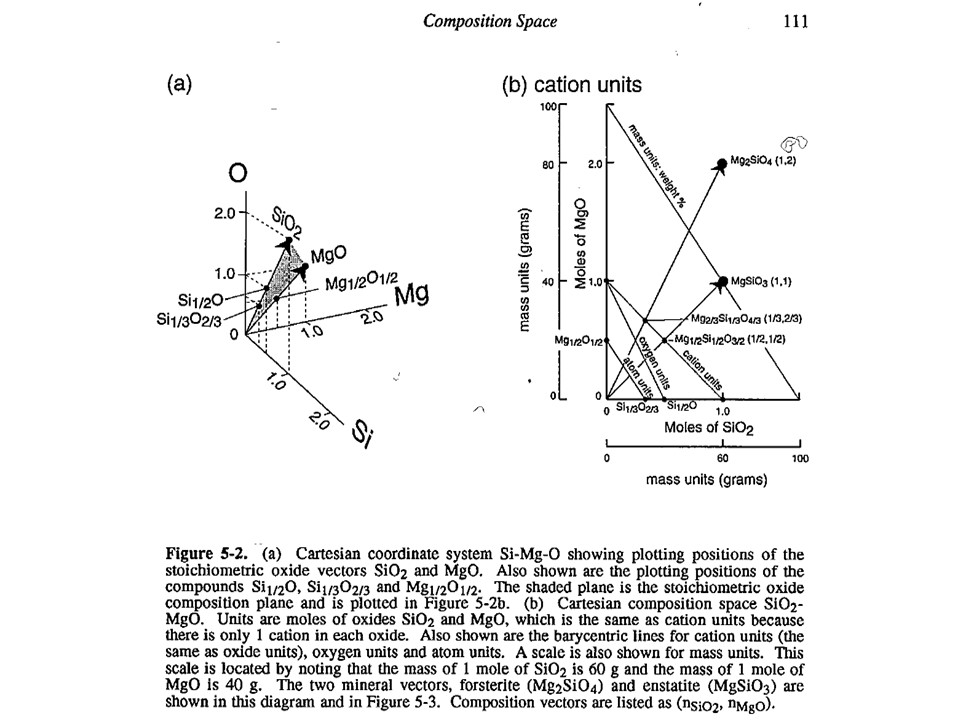
Units of measure. In general, the most commonly used in
metamorphic petrogenesis are moles of oxides. Note, however,
that oxide mass units (wt%) are commonly used in diagrams involving
silicate liquids. In both cases, for historical reasons. Other
interesting units of measure when dealing with oxygen-based
components are oxygen (or oxyequivalent) units, useful for getting
an idea of volume, rather than molecular, abundances of phases
(because oxygen is the largest ion in aluminum-silicates). However,
here we will use moles of oxides only.
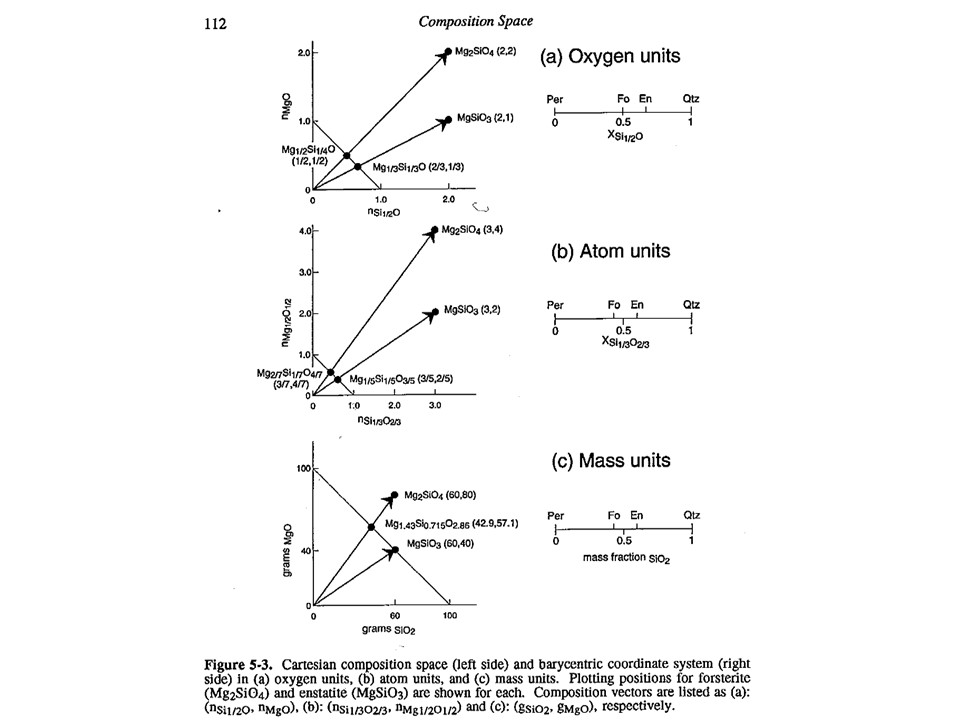
Variations in the baricentric projection as a function of units of
measure.
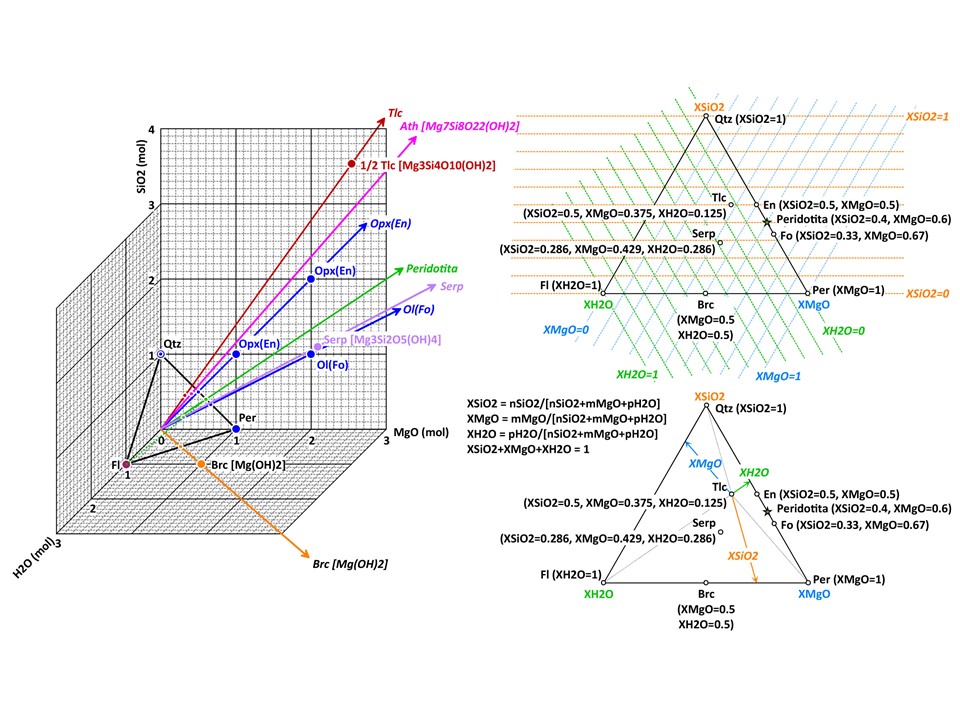
More on oxide molar units in 2 and 3 components systems (4 and
higher component systems are explored below in this document).
Two components:
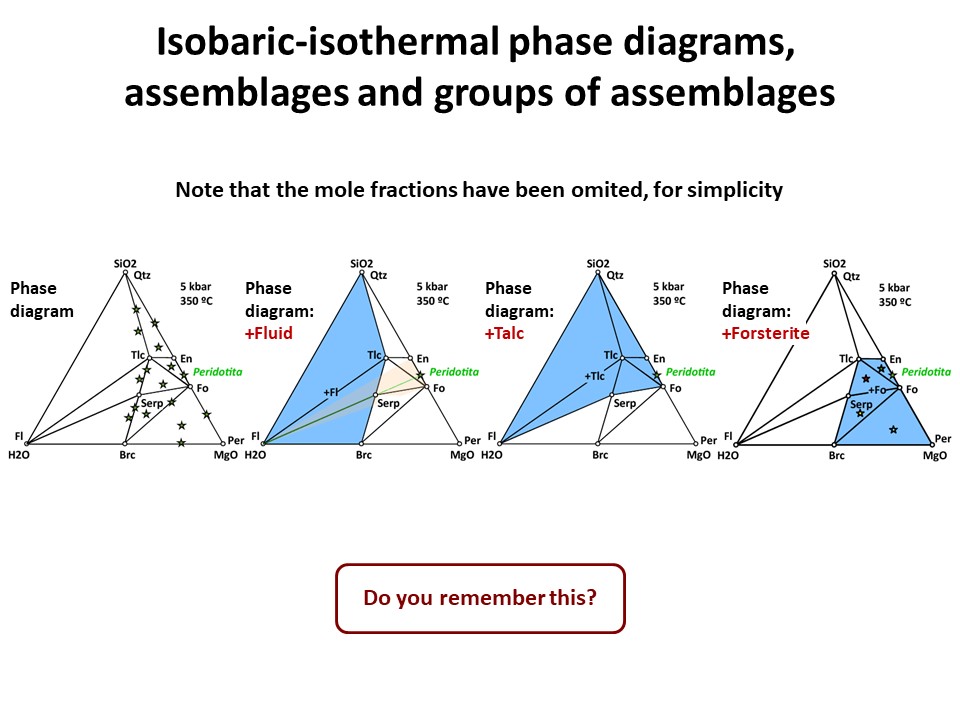
Three components:
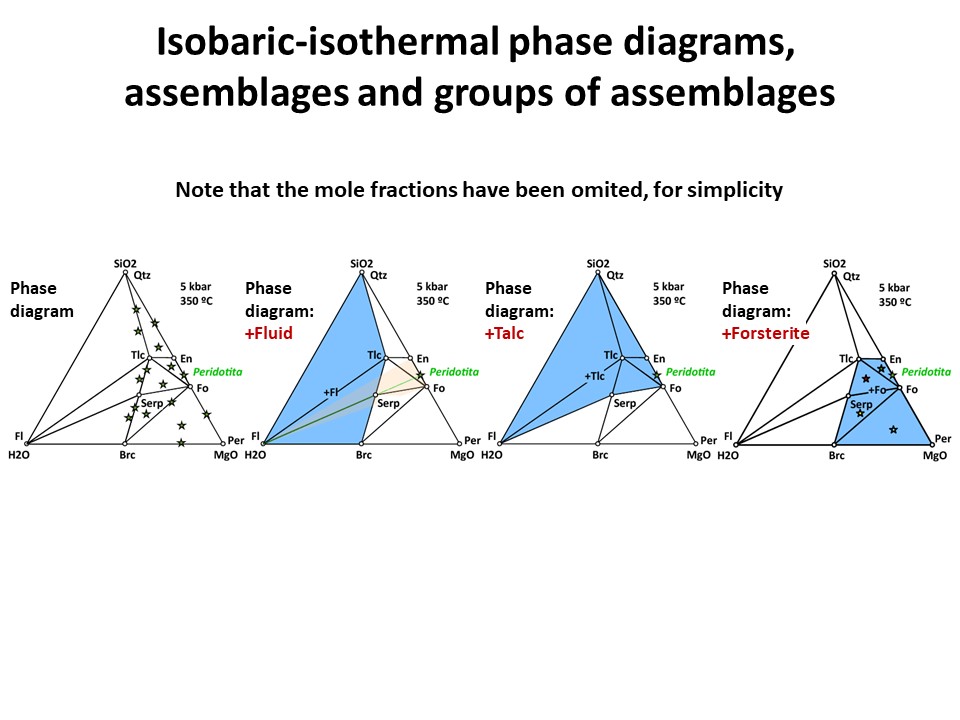
Three components: Phase diagrams with emphasis in subsets of
associations that share a phase (examples in blue below: fluid, talc,
olivine). In these examples, the coexisting minerals are joined by
tie-lines (defining tie-triangles that describe the
stable mineral associations under fixed P-T conditions) and the
stars correspond to the composition of the system (= whole rock
composition).
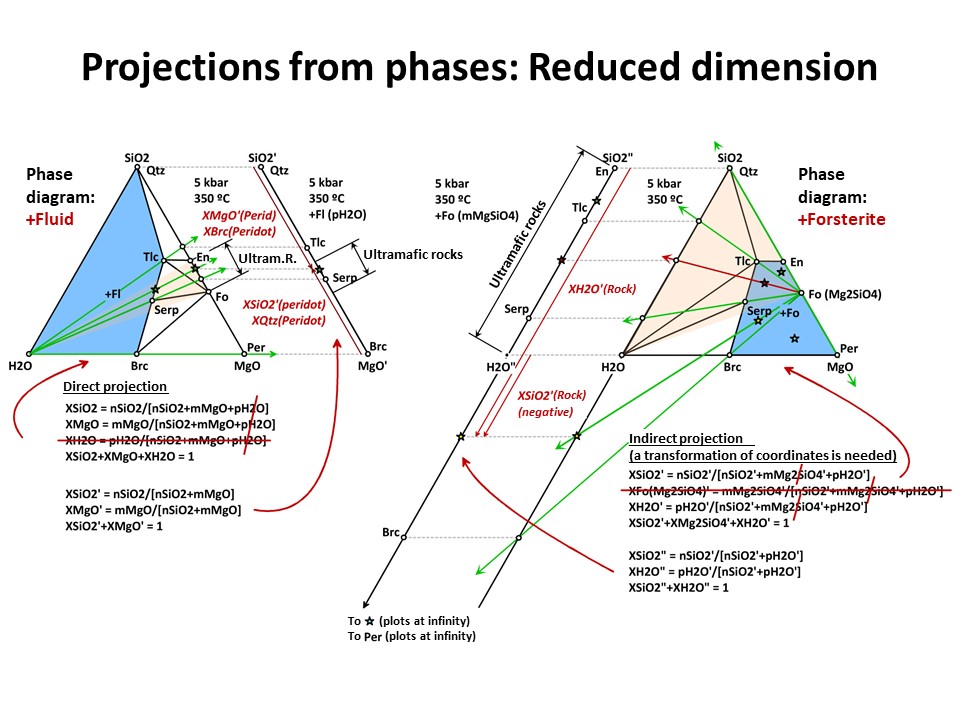
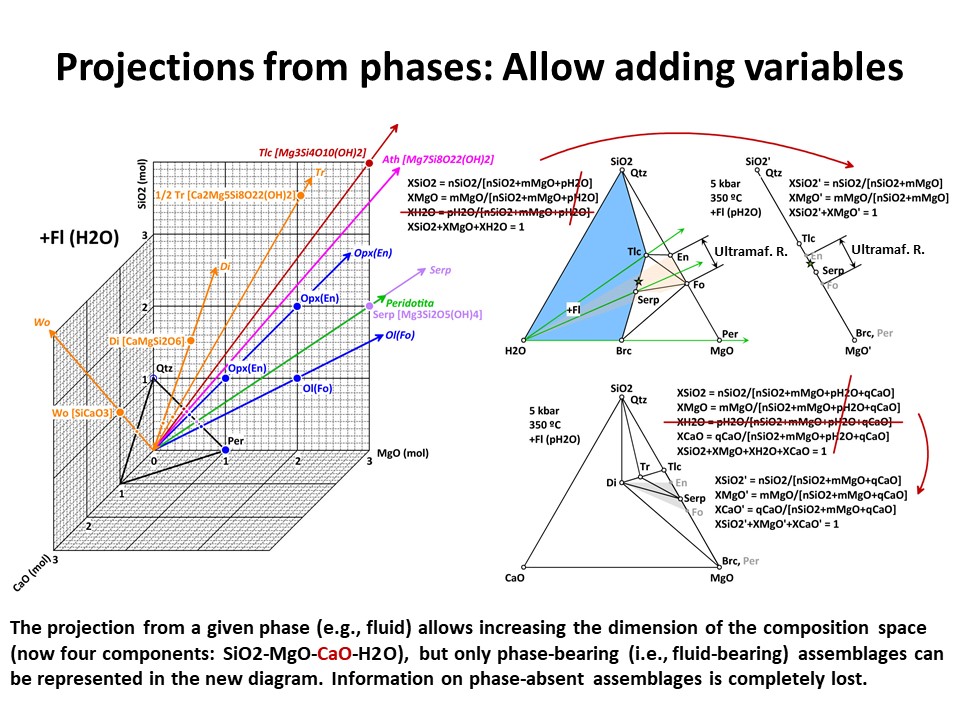
Projections 1
In a given system (say, a 3-component system represented in a
baricentric 2D plane), one can project minerals and rocks from a
given phase onto a part of the system. In doing this, only the
corresponding subset of phase assemblage that share the projection
phase can be projected (examples below, projection from fluid and olivine).
This technique allows reducing the dimension of the represented
baricentric space (which is mandatory when dealing with systems
defined by a large number of chemical variables, as will be shown
below in this document), but information is lost in the process
because only a given subset of phase assemblages are represented in
the projected baricentric space. The information on the amount of
the projection phase is also lost.
The exercise is simple when the projection phase matches the
composition of a component of the system (e.g., quartz = SiO2, periclase = MgO, fluid
= H2O). For the last case, for example, the join (side) SiO2-MgO of
the full SiO2-MgO-H2O triangle can receive the projection. In this
case, the new mole fractions (XSiO2'
and XMgO') are calculated excluding nH2O in the normalization
formulas. This is because the projection from H2O does not impact in
the amounts of nSiO2 (= nSiO2') y nMgO (= nMgO') of the
projected chemical species. The same holds for projections from
quartz (onto MgO-H2O join) or periclase (onto the SiO2-H2O join),
which respective mole fractions are calculated excluding nSiO2 or nMgO,
respectively).
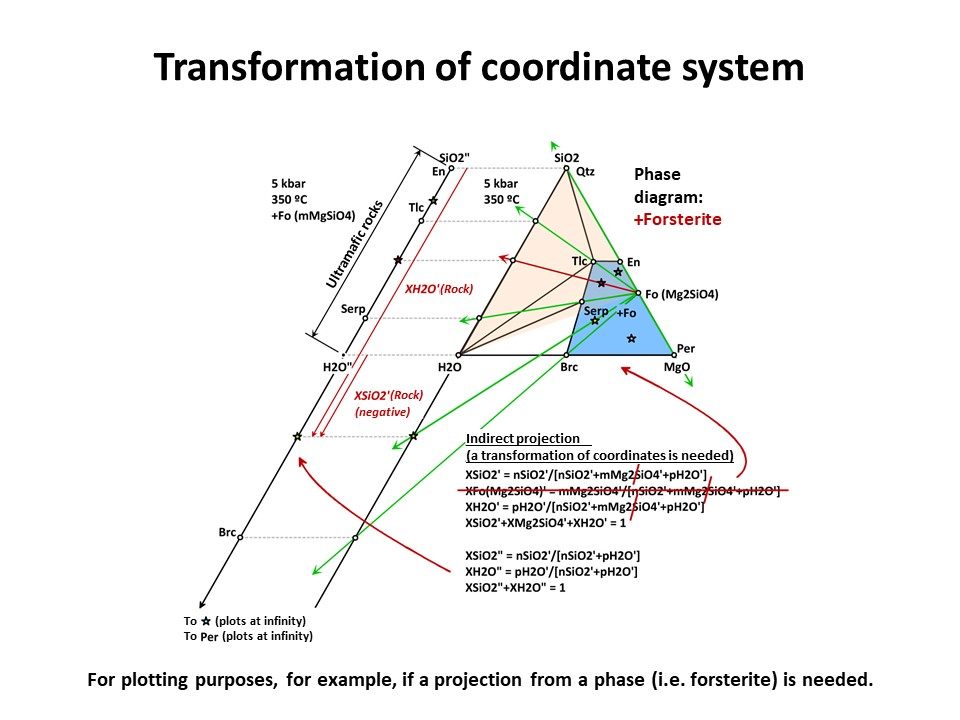
But the exercise is not son simple when the projection phase is more
complex. Besides graphical solutions such as the right side
diagram of the following figure, which represents a projection from
olivine (forsterite) onto the SiO2-H2O join, a quantitative method
is generally needed for calculating the new mole fractions (XH2O" y
XSiO2") because no longer the procedure involves normalization using
the original chemical variables. (->
see coordinate transformation, treated
below). It must be noted that, for
any species with SiO2+MgO, the projection from forsterite (that
bears SiO2+MgO in the proportion 1:2) implies that nSiO2" and nMgO"
do not equal nSiO2 y nMgO in the species. The latter must be
corrected before normalization "discounting the amounts of
forsterite in the species" in the proportion SiO2:MgO = 1:2. For
this reason, the values of XSiO2" of some species are negative
(i.e., plot at the negative sector of SiO2", away the locus of H2O",
in the SiO2"-H2O" join) and even some species (such as periclase)
plot at infinity (away the locus of H2O"), as shown in the figure.
This problem will be treated quantitatively below. Meanwhile, we
will inspect the graphical solution shown below in detail.
The projection of phases and rocks from a phase on a given subsystem
(e.g., join) allows considering the graphical representation of
larger systems, making the representation be closer to natural
systems, but only under the limiting condition that all the
projected assemblages must contain the projection phase. The
assemblages that do not contain the projection phase H2O fluid in
the following figure have been drawn in grey. In the case
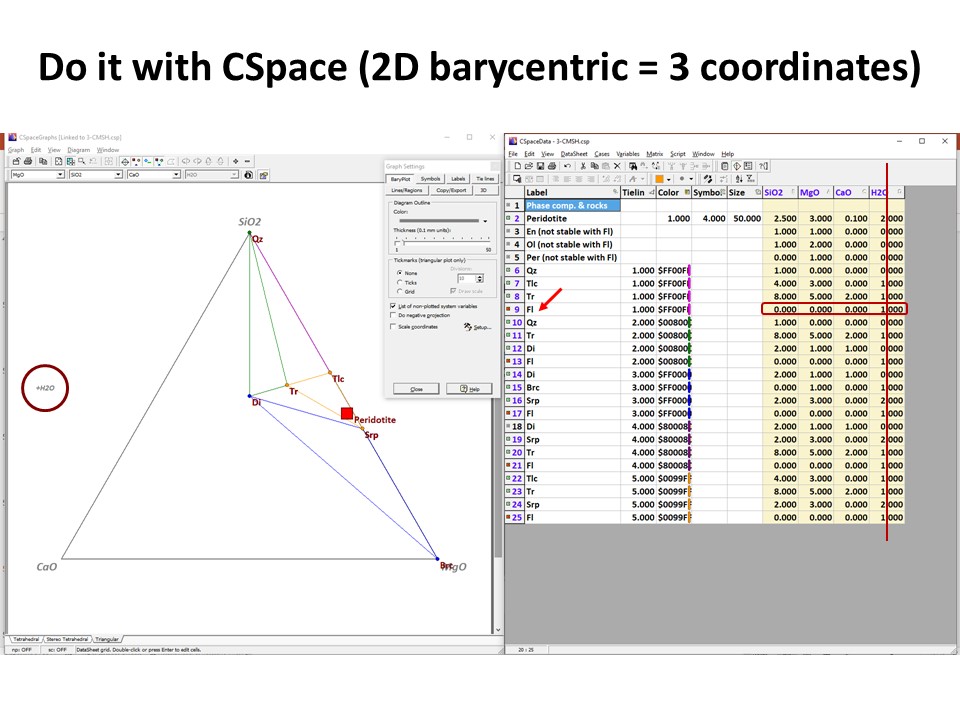
illustrated below, the system enlarges up to 4 components: SiO2-MgO-CaO-H2O.
Because it is projected from H2O fluid, the simplest new ternary
diagram MgO-SiO2-CaO (the composition of ultramafic rocks are
represented by the grey shaded region defined by Fo-En-Di in the
ternary diagram MgO-SiO2-CaO):
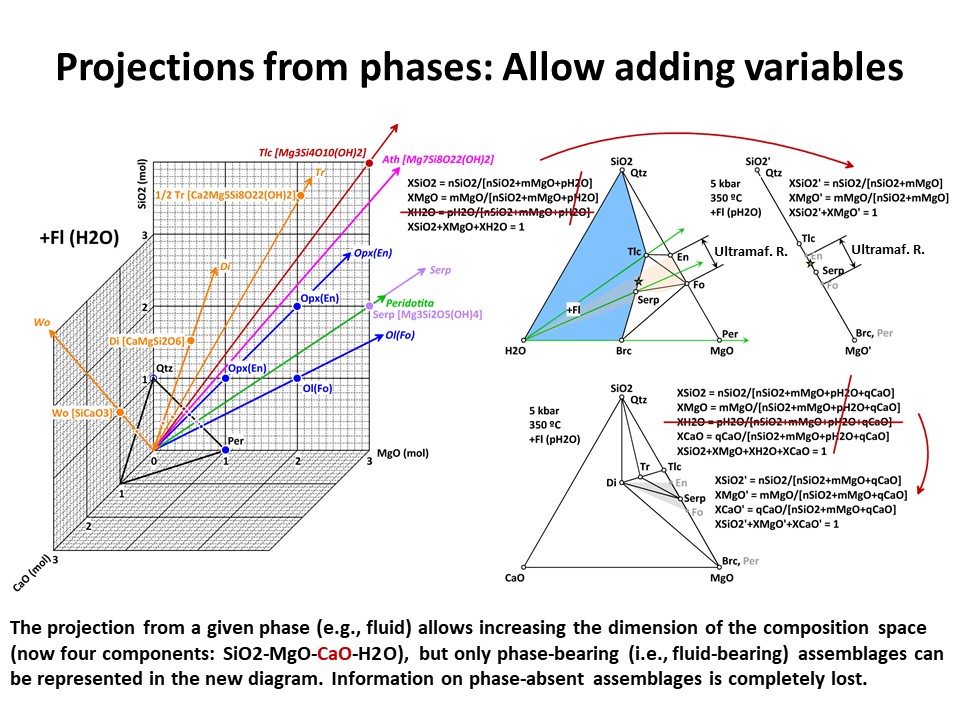
In the former case, the calculation of the new mole fractions is
simple because the composition of the projection phase matches the
composition of one of the chemical components that define the
system. But, as above, this is not so in case of projections from
complex phases, like forsterite (->
Coordinate transformation).
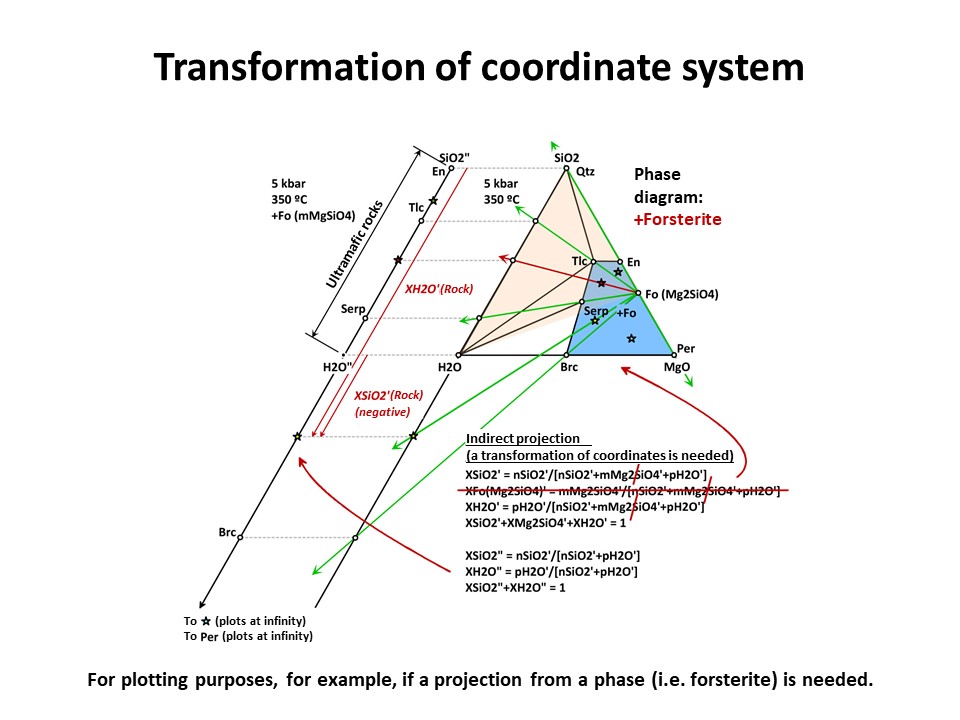
Coordinate transformation is needed for many other purposes (not
only the graphical representation of systems with increased number
of components). For example, the calculation of the (molar) abundance
of minerals (or, in general, species) in a rock. Below, you will
find examples in binary a ternary systems though, again, the
principles are identical for n-dimension systems. The question is:
Provided that we now the composition olivine (forsterite),
orthopyroxene (enstatite) and a peridotite in the SiO2-MgO system,
how much olivine and orthopyroxene does the peridotite have?
Provided that we now the composition olivine (forsterite), talc,
serpentine and an hydrated peridotite in the SiO2-MgO-H2O system,
how much olivine, talc, serpentine does the peridotite have at 350
ºC and 5 kbar?
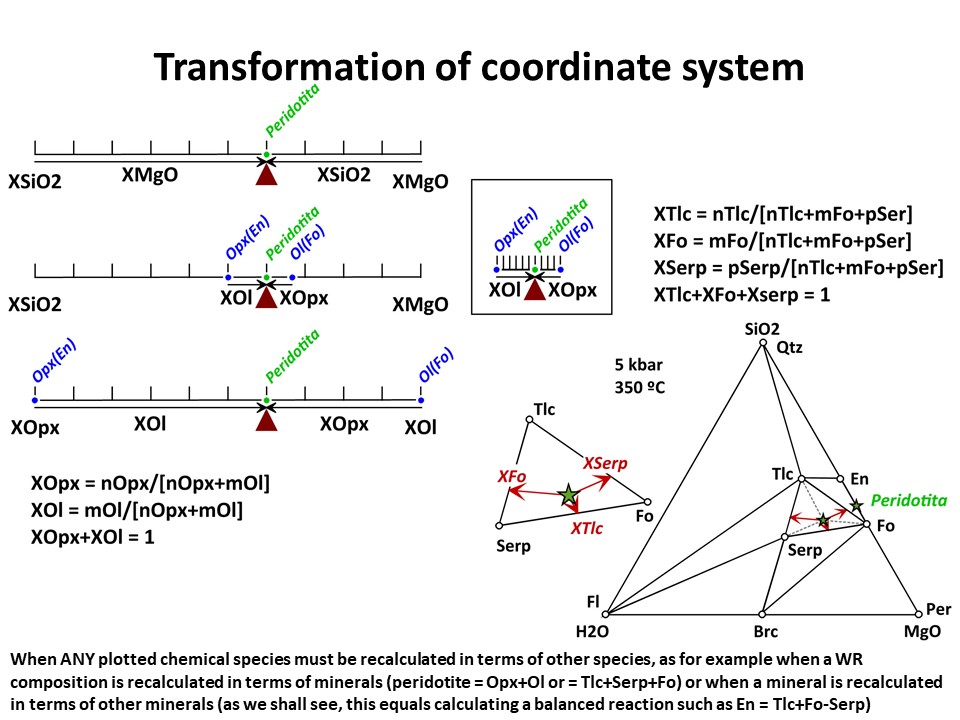
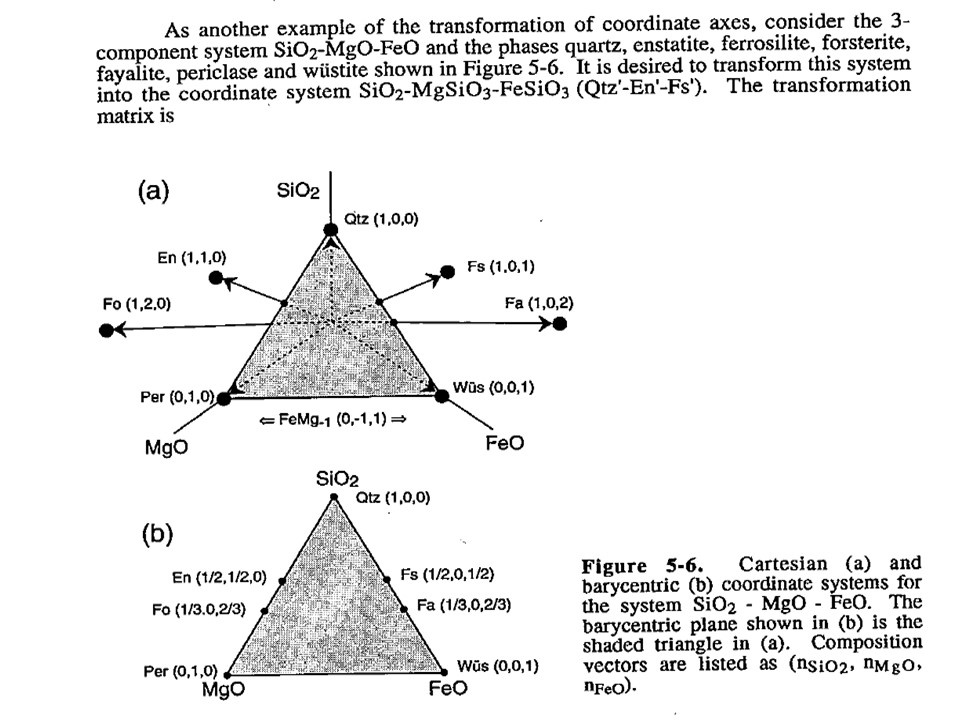
Coordinate transformation
Coordinate transformation is a rather common algebraic problem that
make use of matrix calculus. Coordinate transformation has many
applications in Mineralogy,
Petrology and Geochemistry. It is known as "base transformation",
"component transformation" or "lineal mapping".
* The issue is to express a chemical species (mineral, rock, fluid,
melt...) in terms of a set of new components given its known
composition in terms of a set of old components. This can be
achieved solving a set of linear mass-balance equations.
As a general rule, the number of old and new components and, hence,
the number of equations, must be equal. In the set
of equations, there is a mass-balance per old component, so that the structure
of the equations is regular and can be represented as in matrix
notation, as below using matrix inversion among a number of ways of
solving the set of equations.
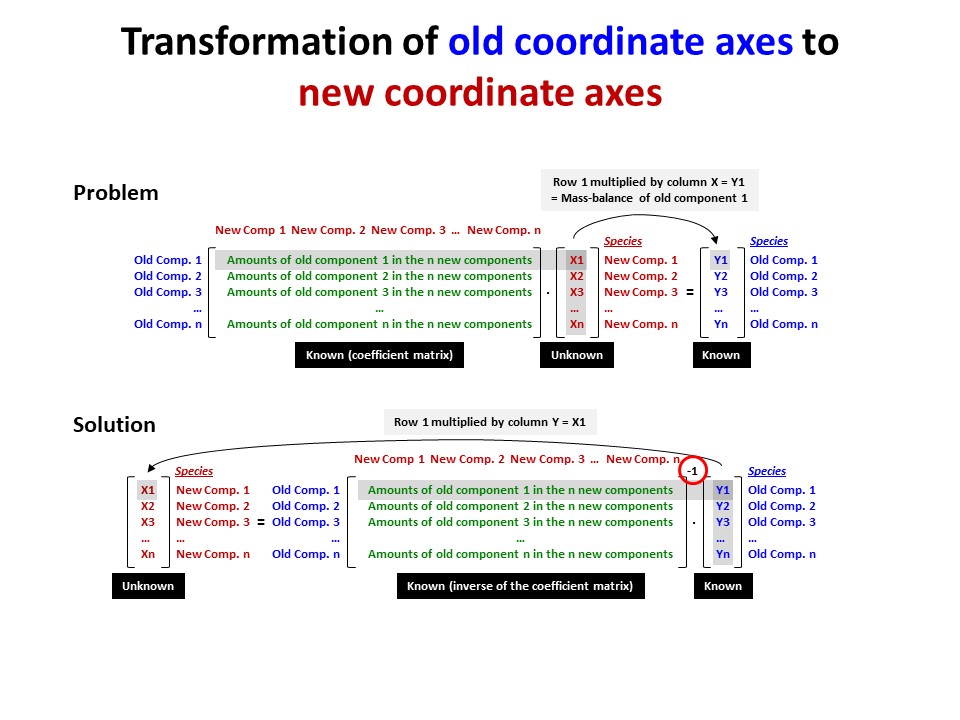
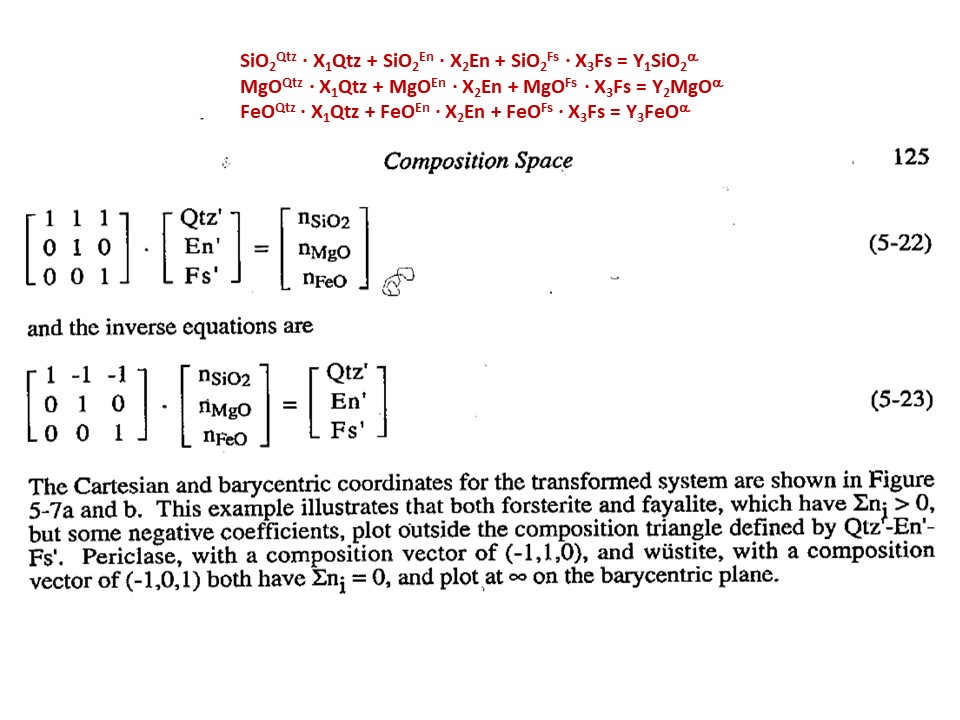
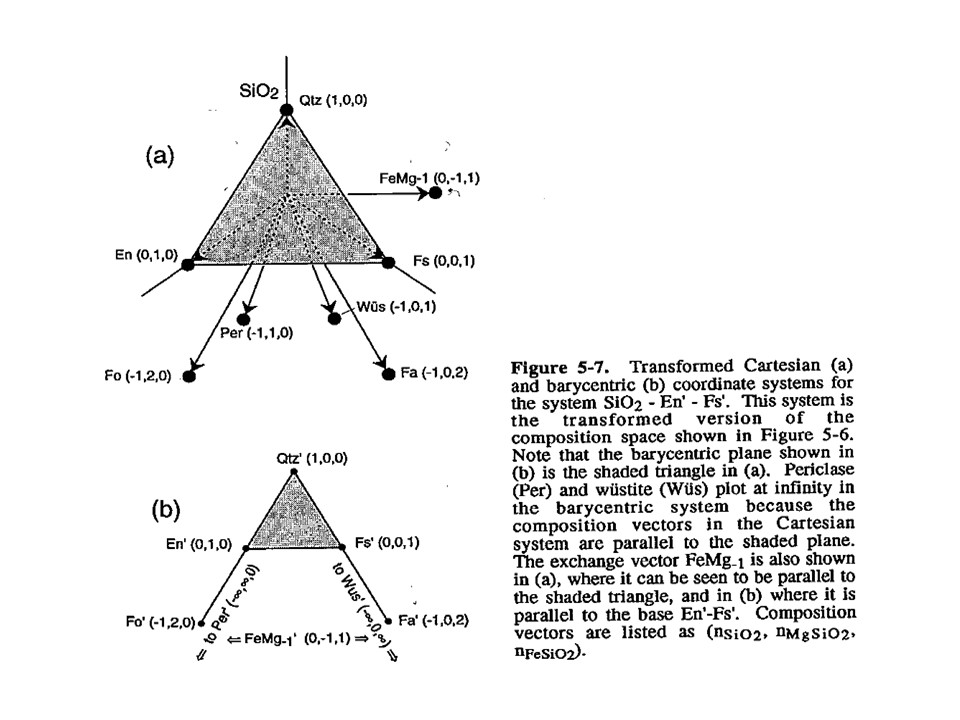
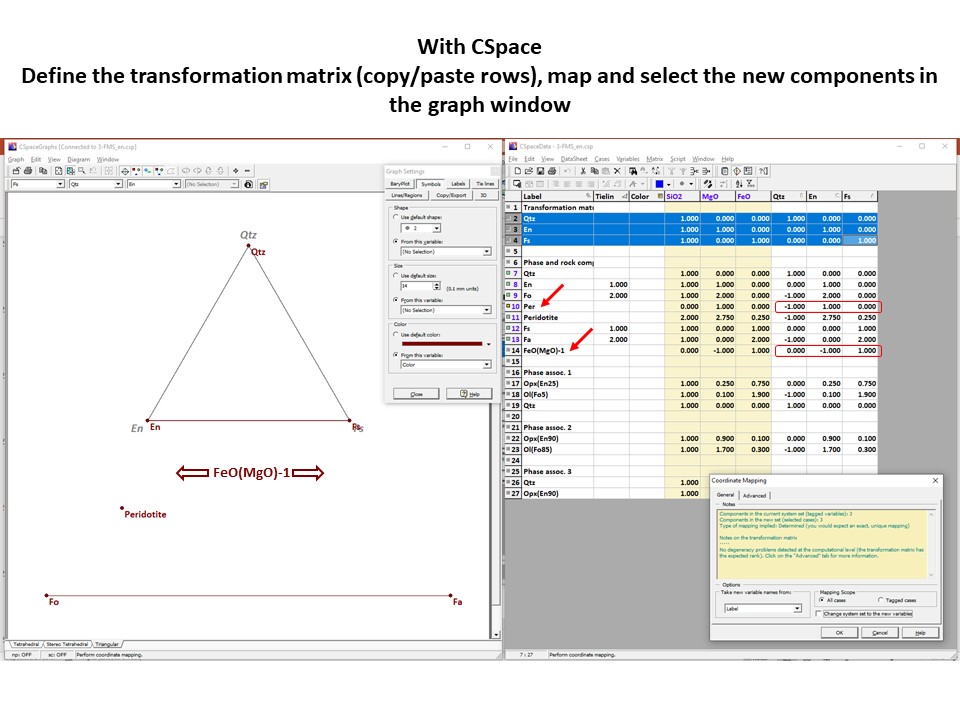
Let's consider the issue in the simple SiO2-MgO system.

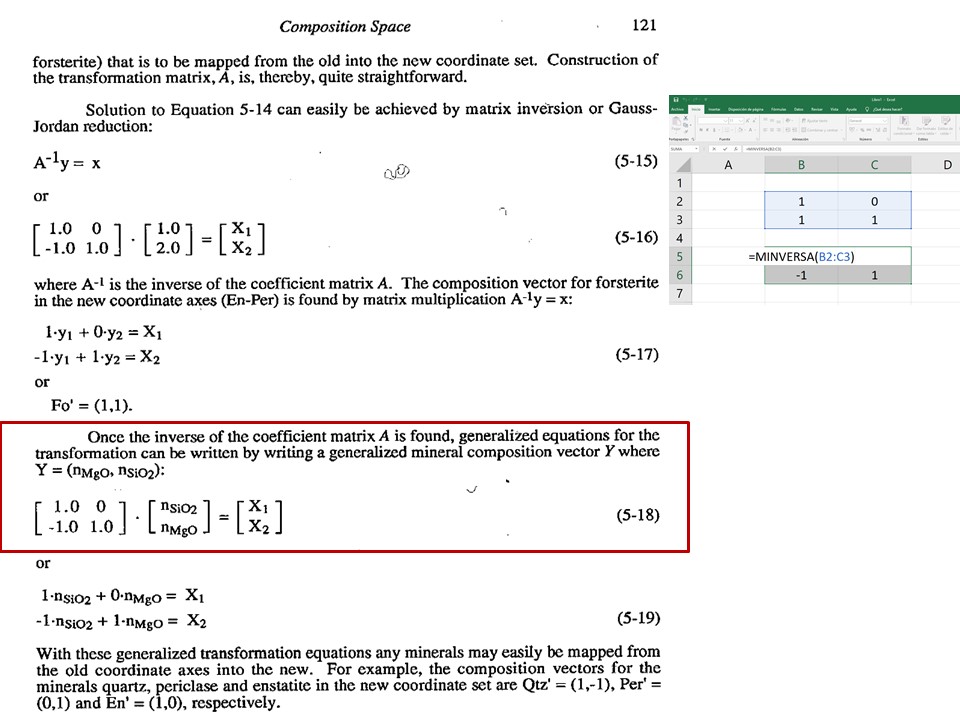
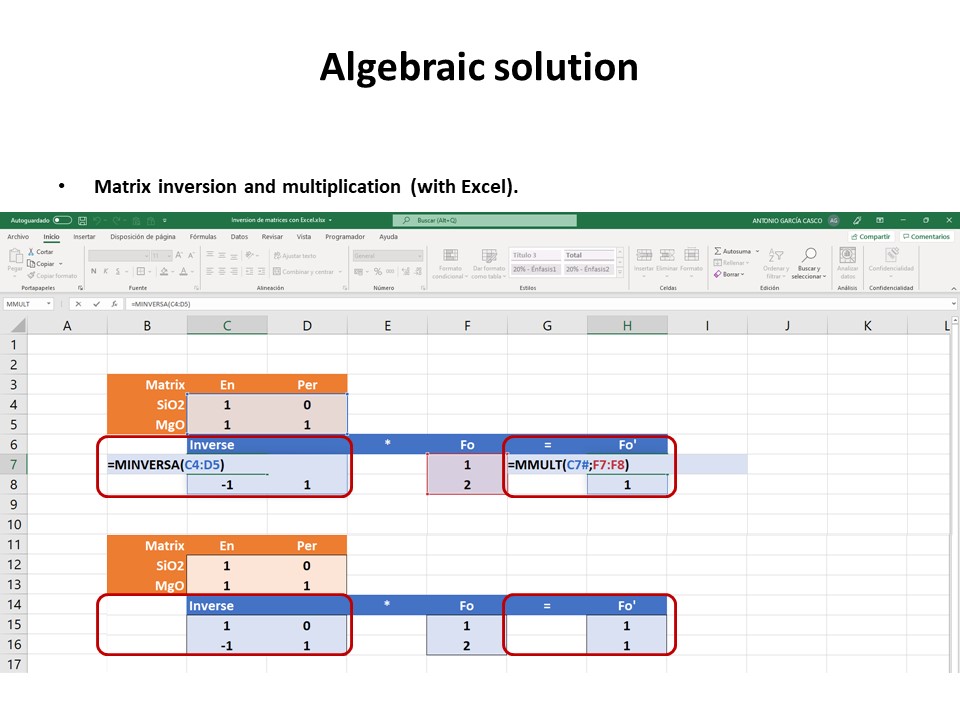
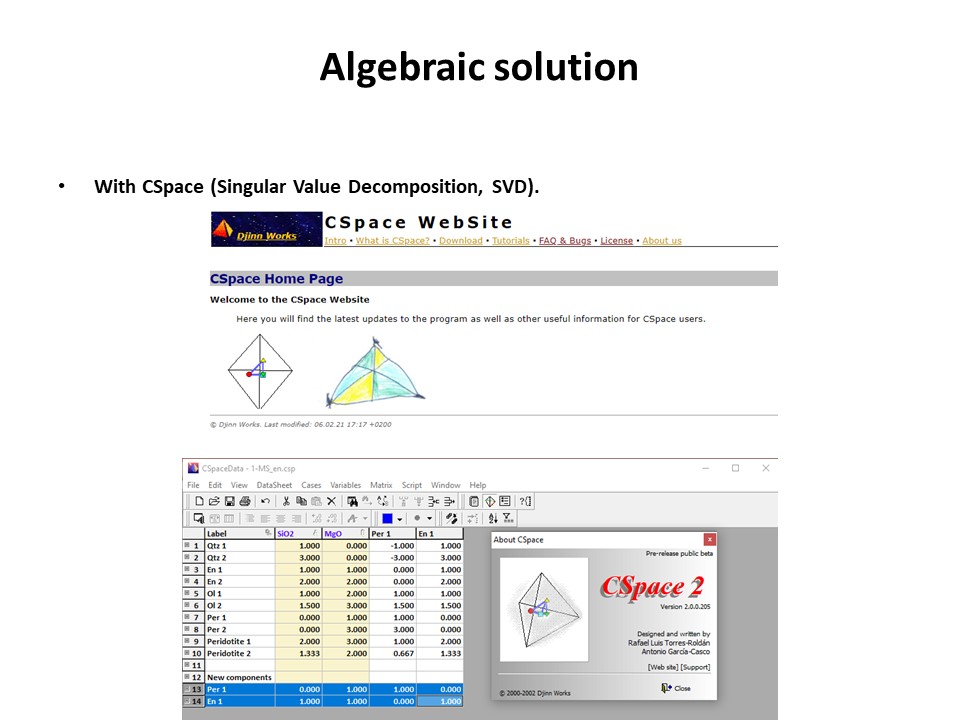
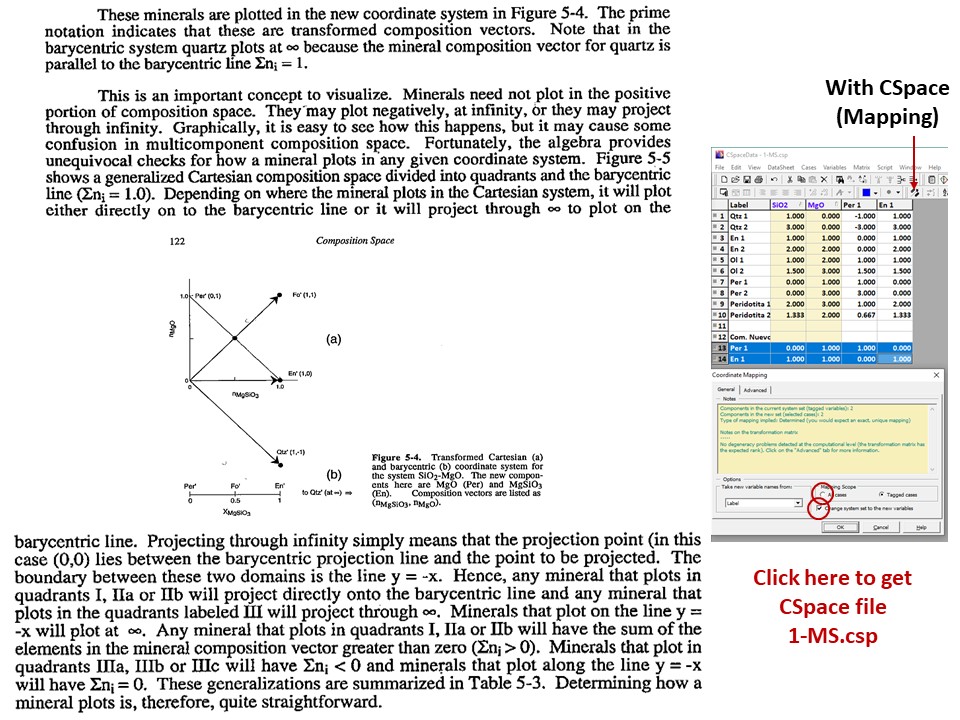
Or click here: 1-MS.csp
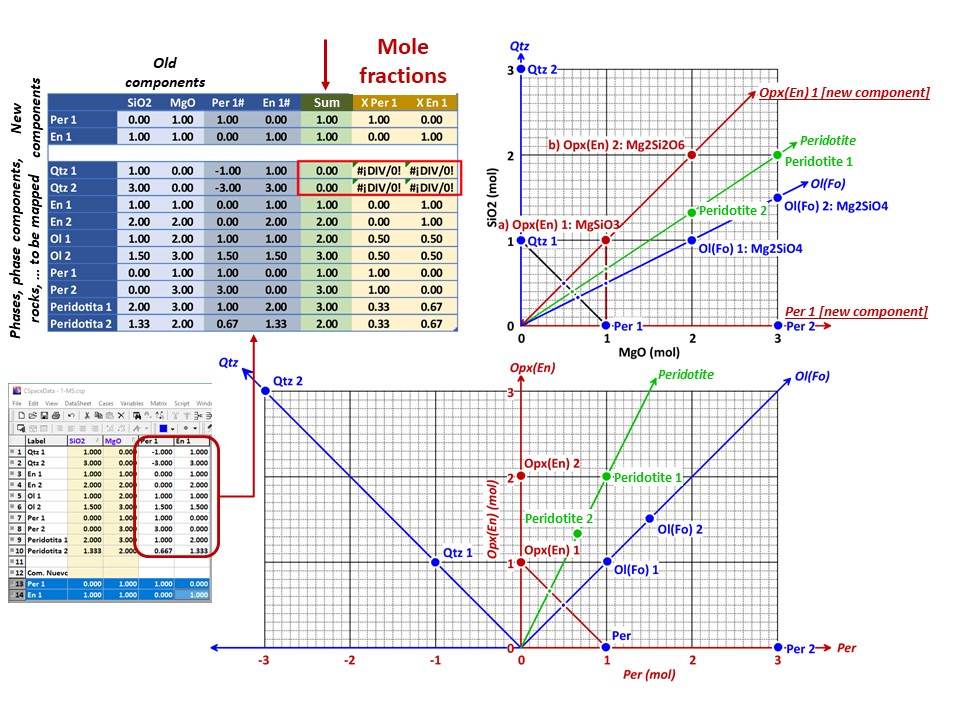
Coordinate transformation: SiO2-MgO -> En(SiMgO3)-Per(MgO).
Note that quartz projects at infinity (that is, it cannot be
projected) in the new coordinate system.
Coordinate transformation: SiO2-MgO -> En(Si2Mg2O6)-Per(MgO).
Note that quartz projects at infinity (that is, it cannot be
projected) in the new coordinate system, but it can be projected
through infinity with a negative value of XEn (that is, it can
be projected in the negative sector of XEn)
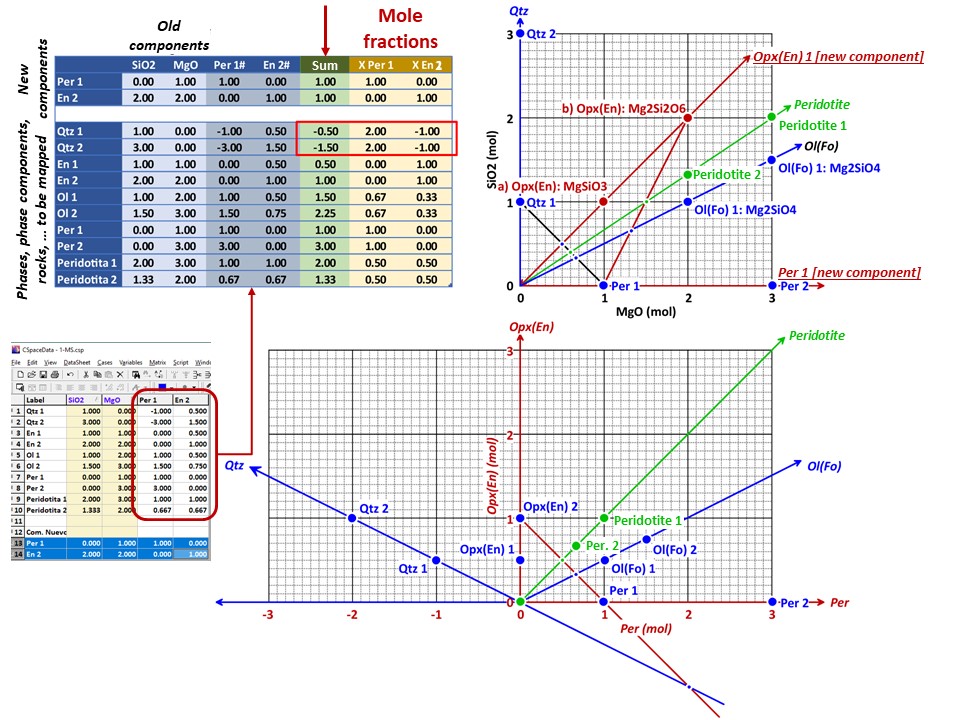
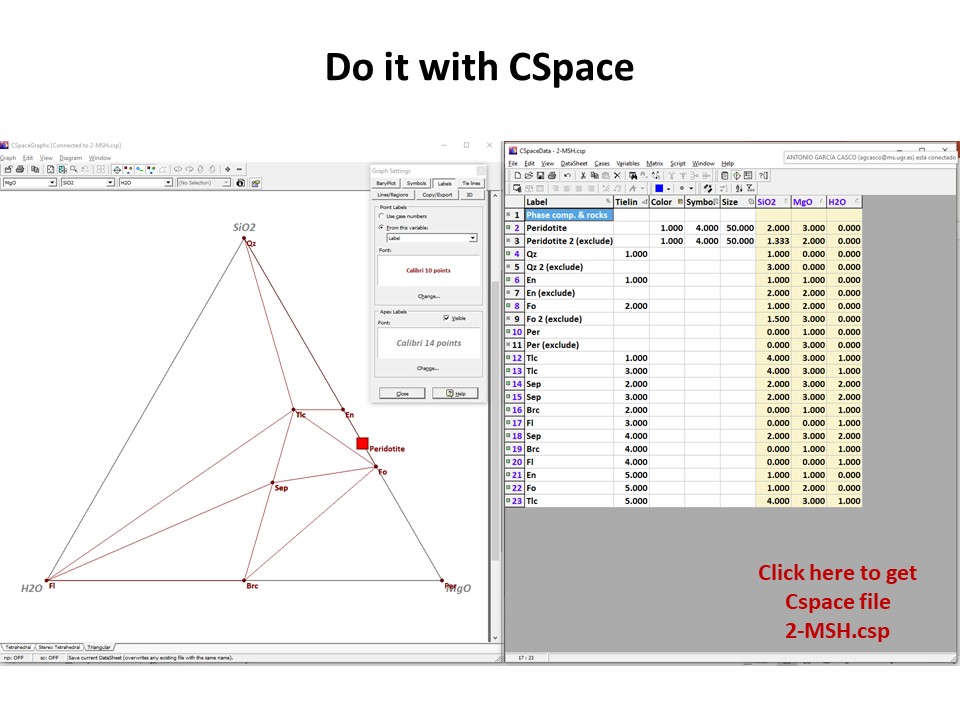
Or click here: 2-MSH.csp
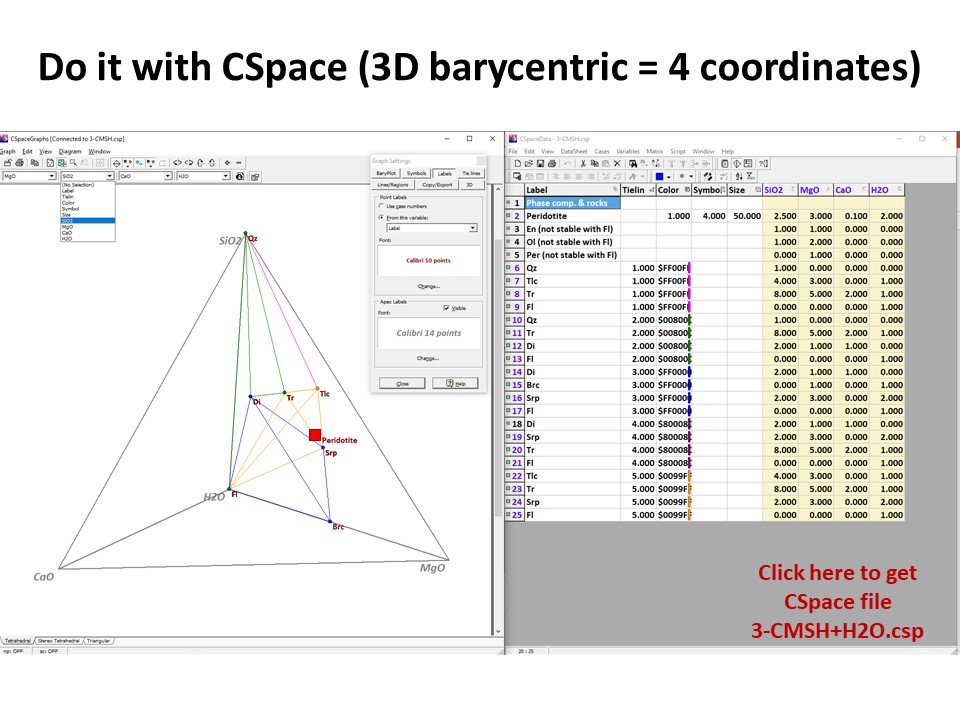
Or click here: CMSH+H2O.csp
Or click here: 3-CMSH+Fo.csp
Or click here: 4-FMS.csp
Projection
and condensation of the system

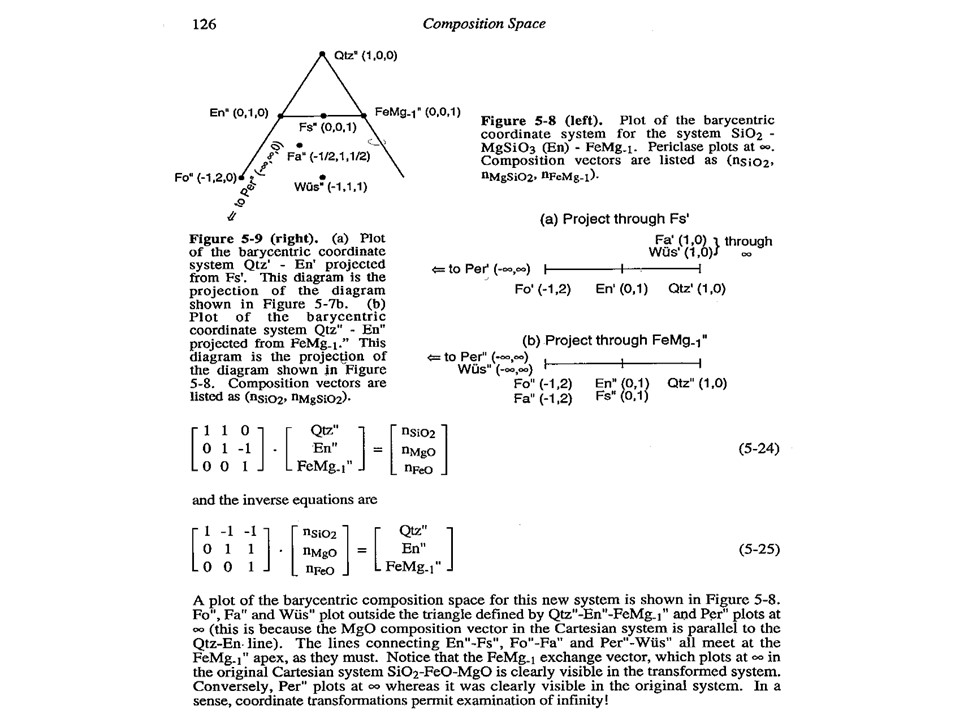
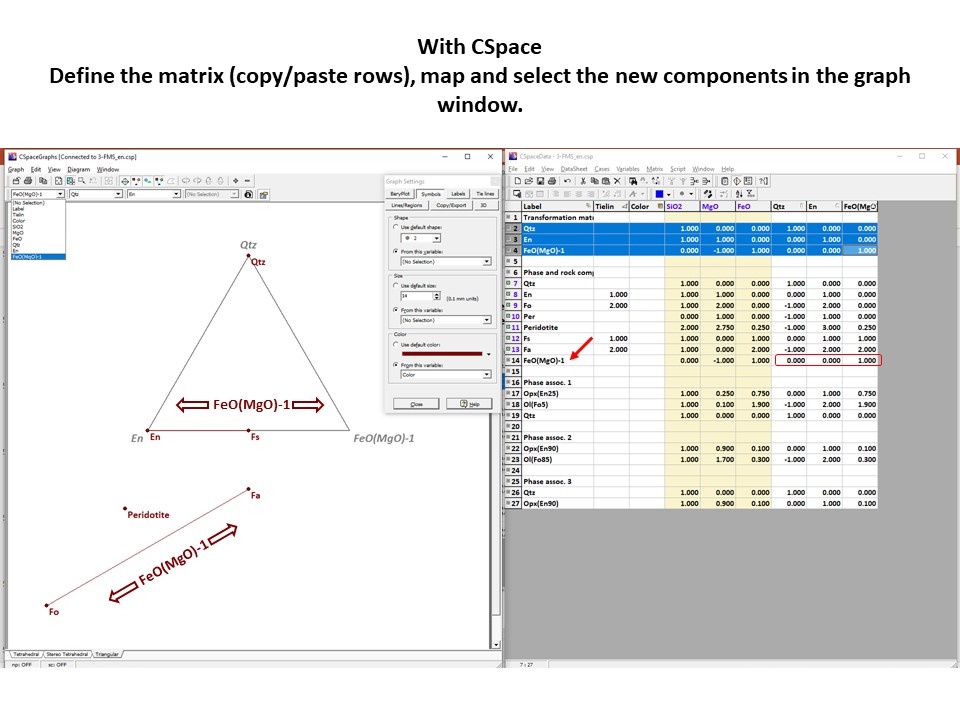
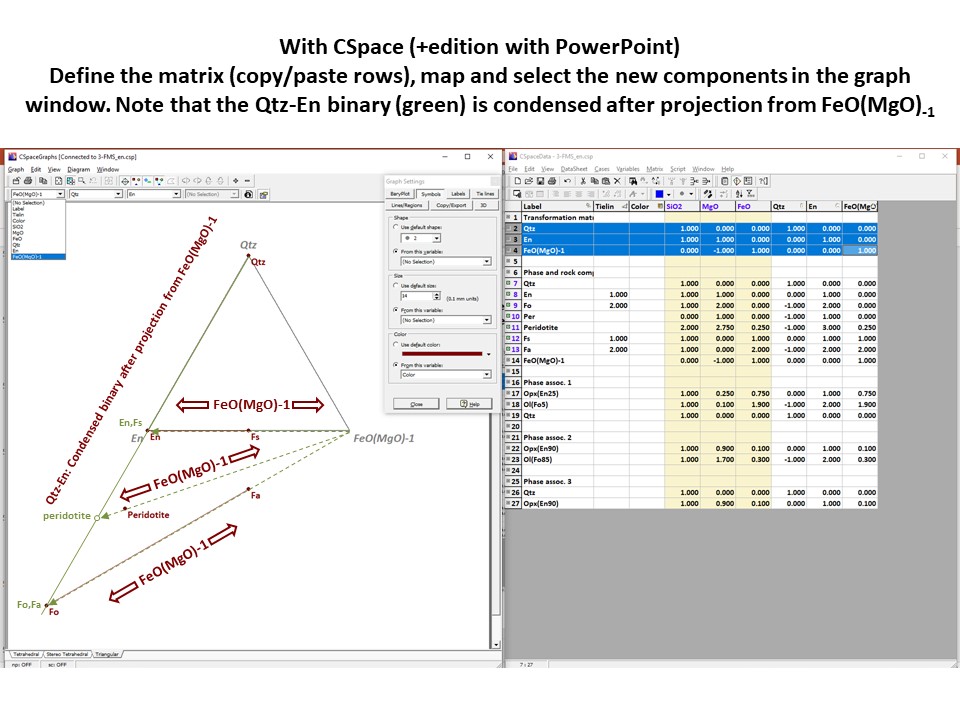
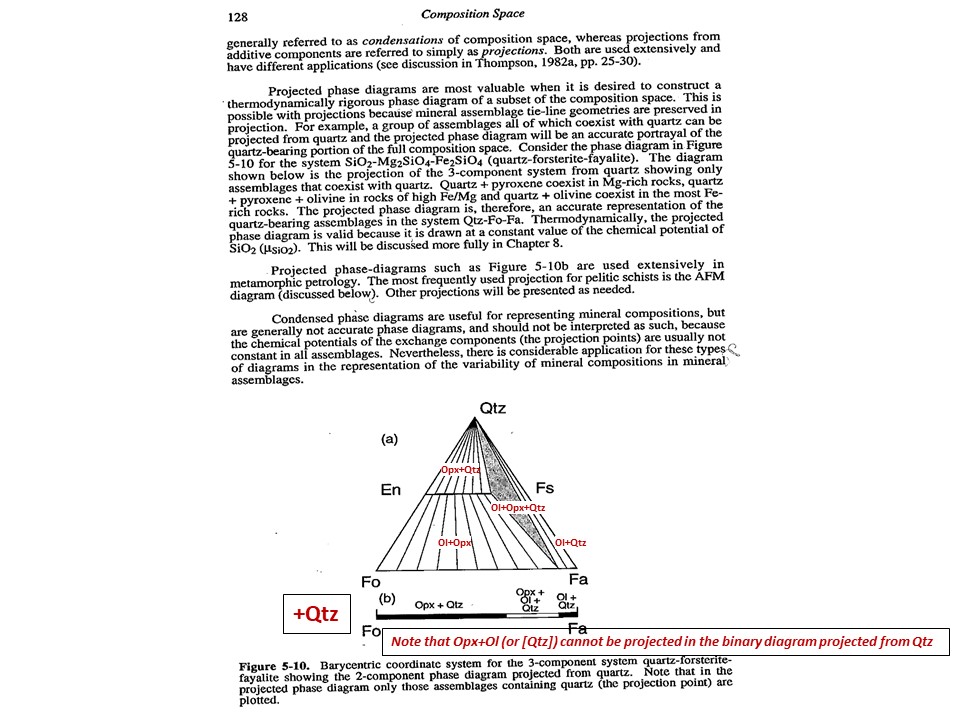
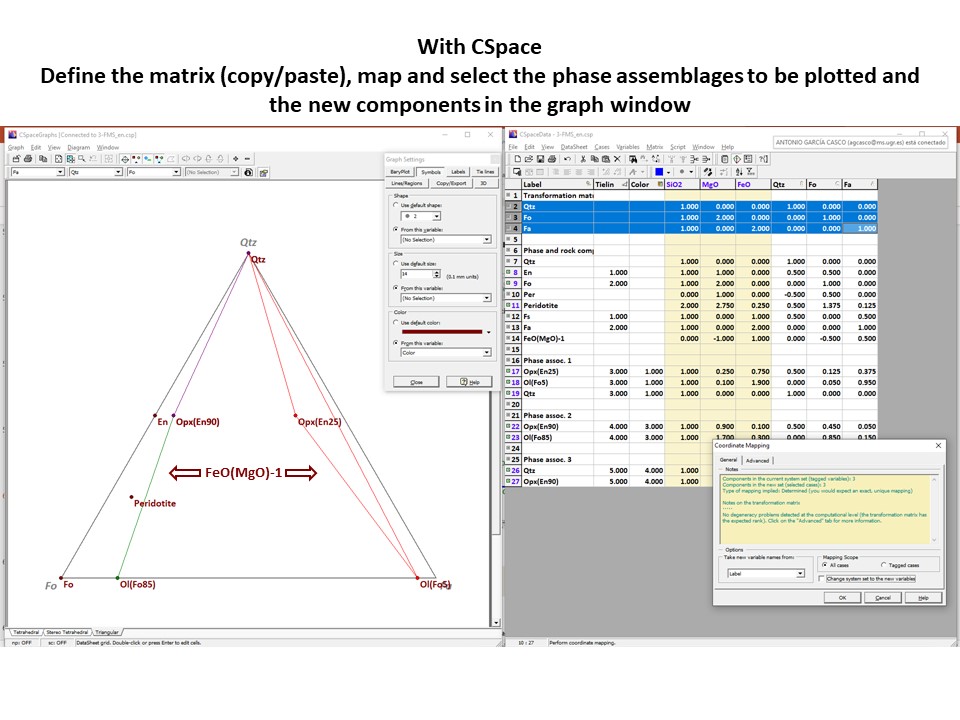
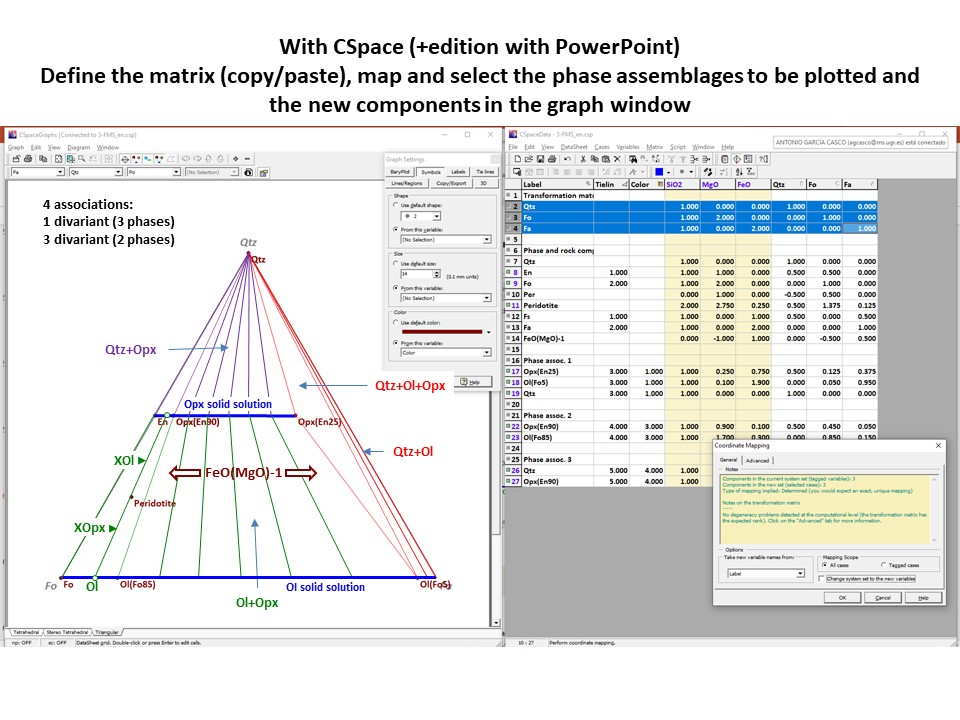
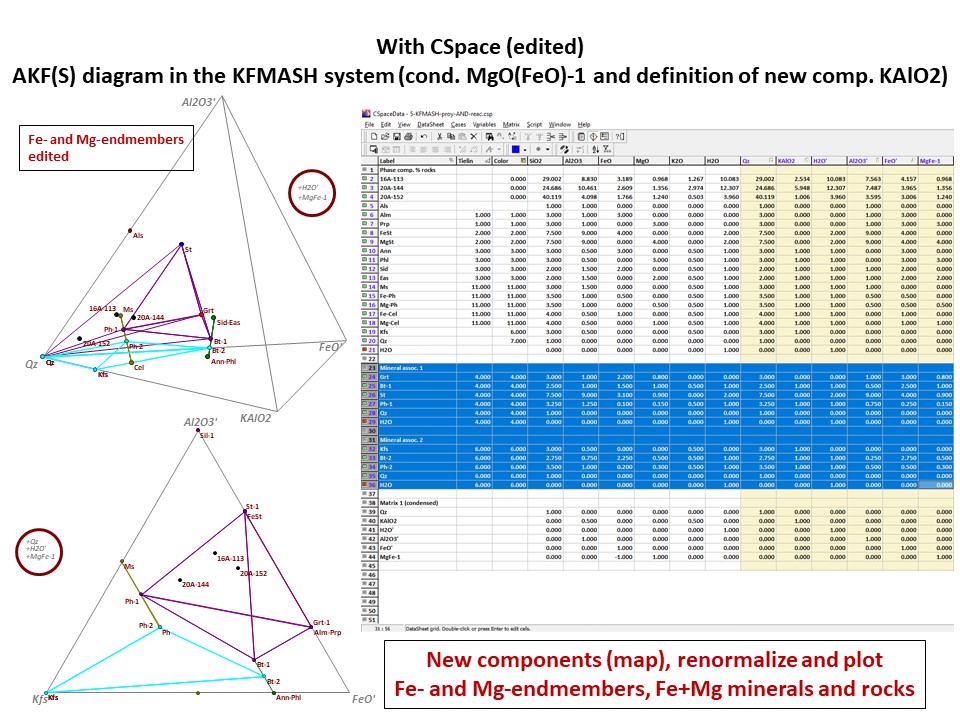
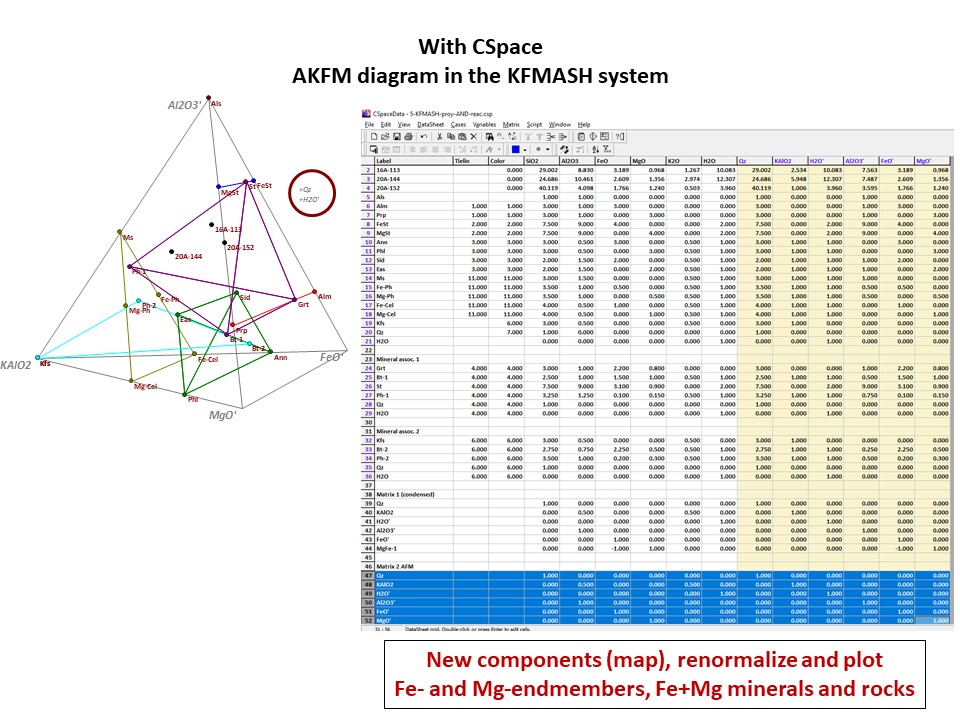
Diagrama AKF

Prof. Stephen A. Nelson. Tulane University:
https://www.tulane.edu/~sanelson/eens212/triangular_plots_metamophic_petrology.htm
Amberlynn Kristin Park:
https://slideplayer.com/slide/13190833/
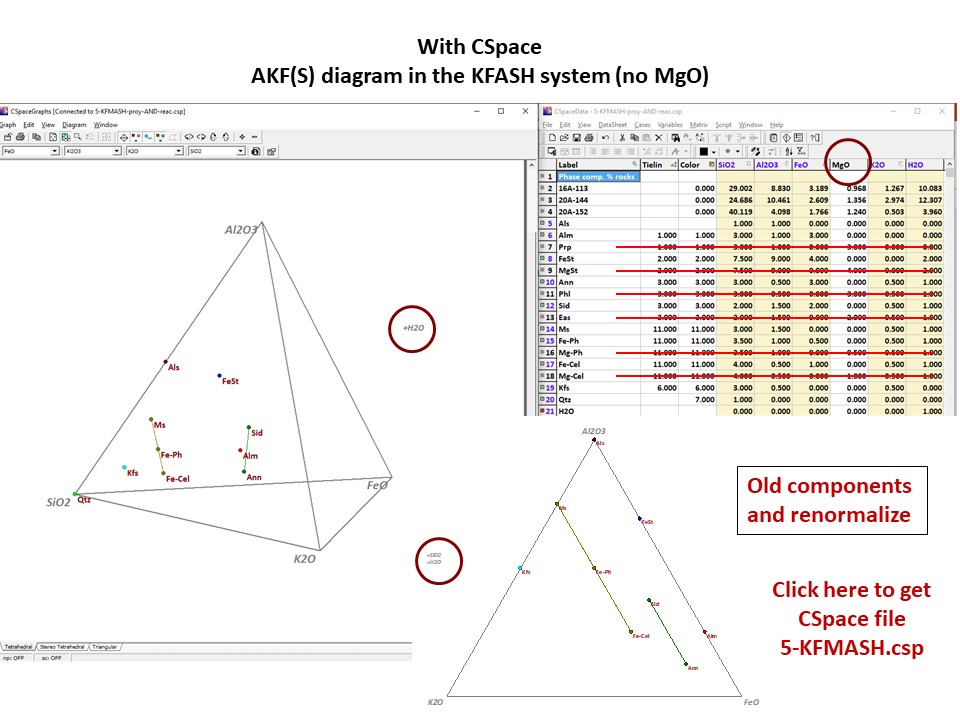
Or click here: 5-KFMASH.csp
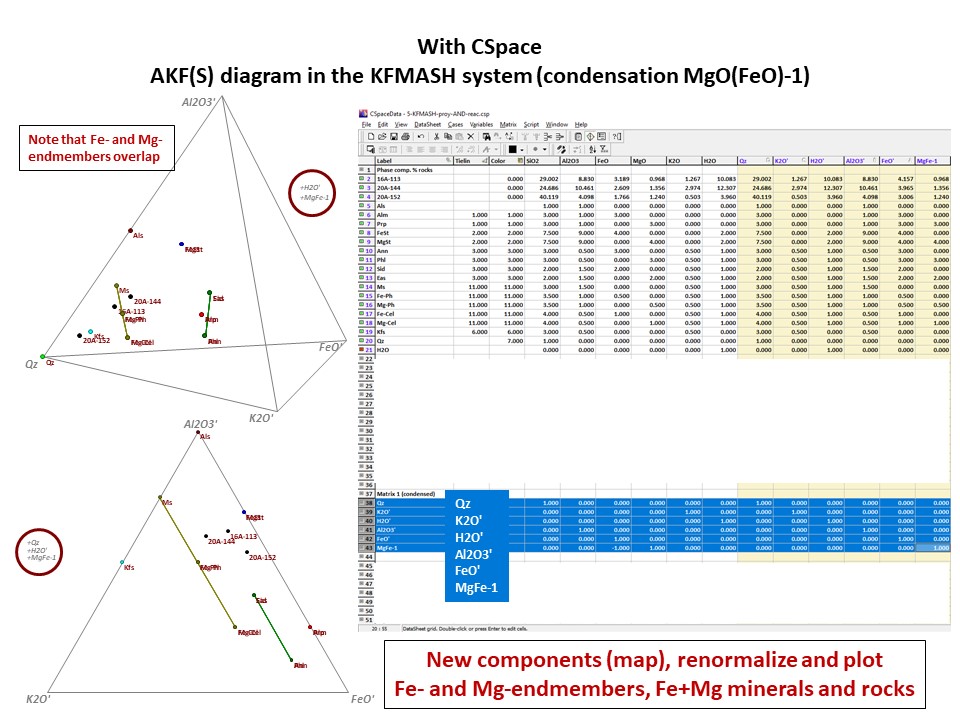
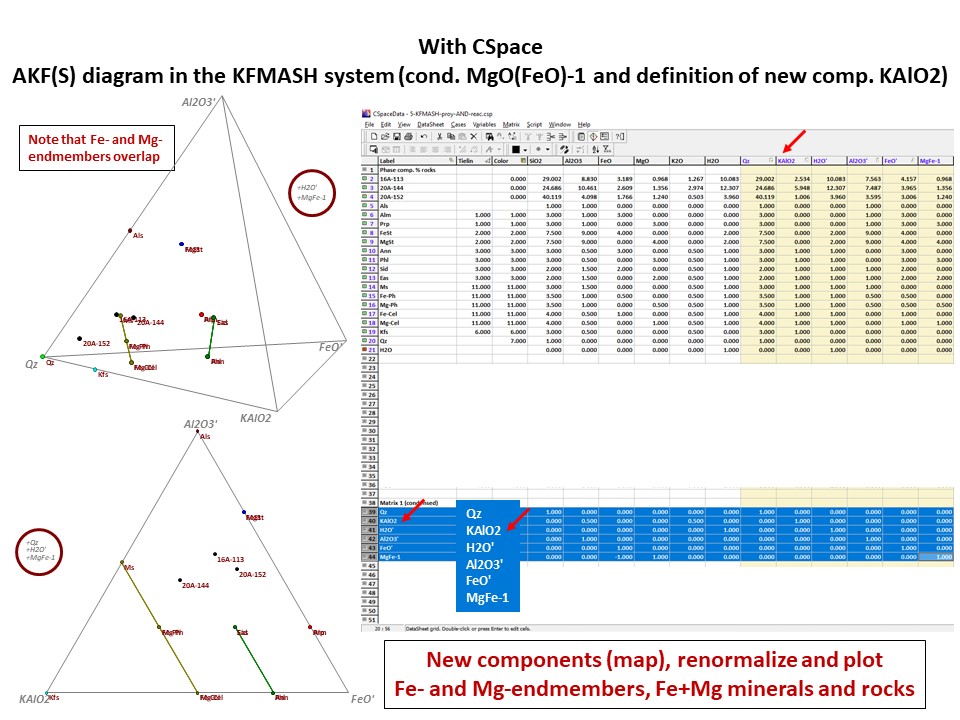
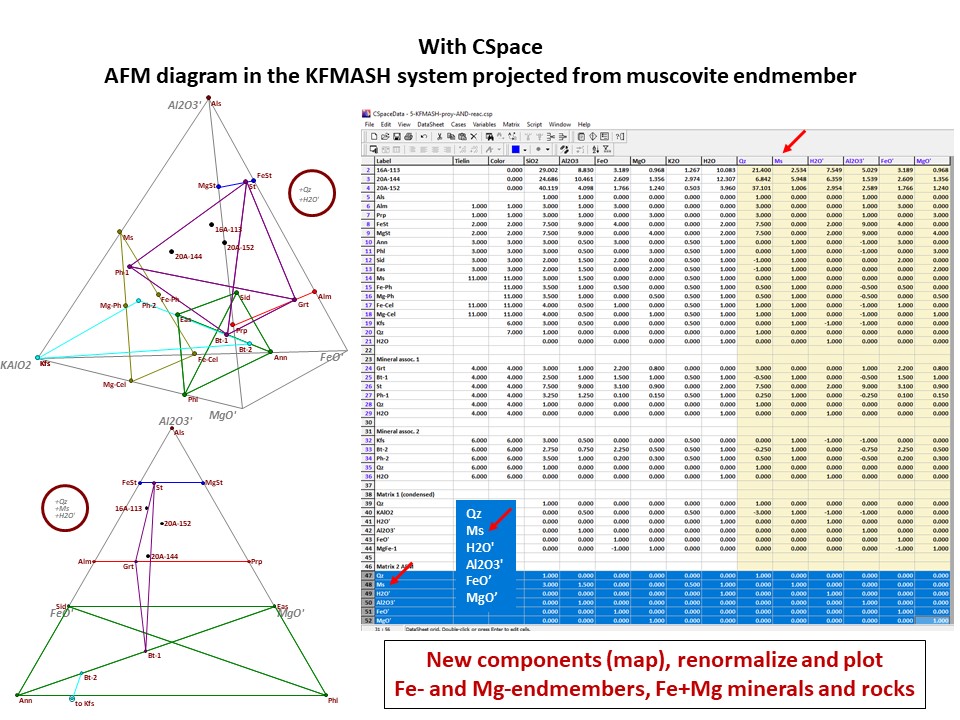
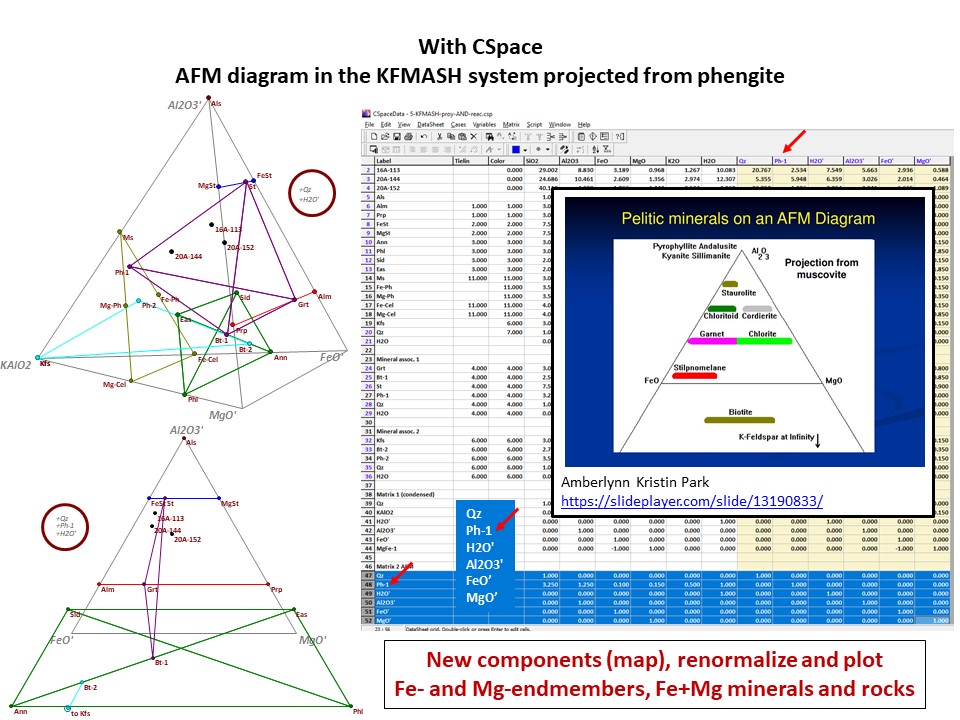
AFM diagram

Prof. Stephen A. Nelson. Tulane University:
https://www.tulane.edu/~sanelson/eens212/triangular_plots_metamophic_petrology.htm
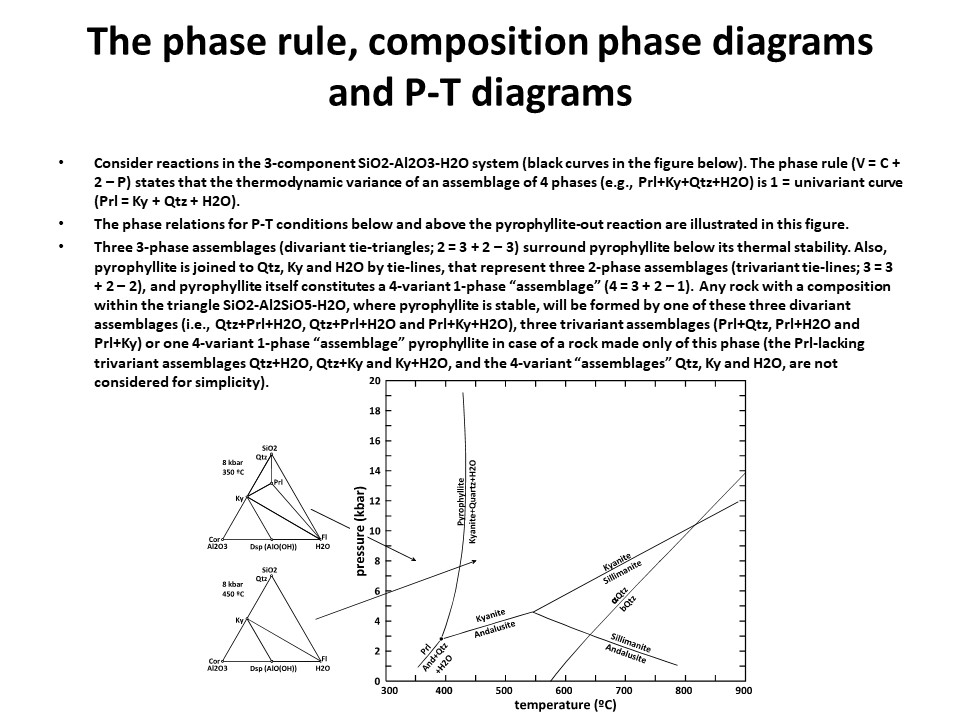
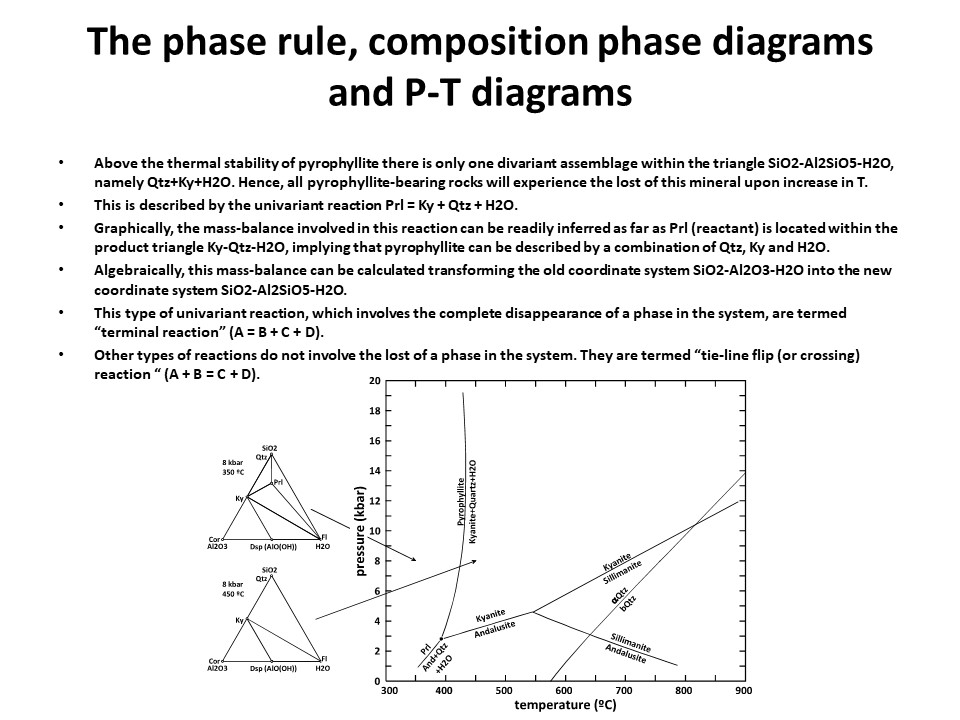
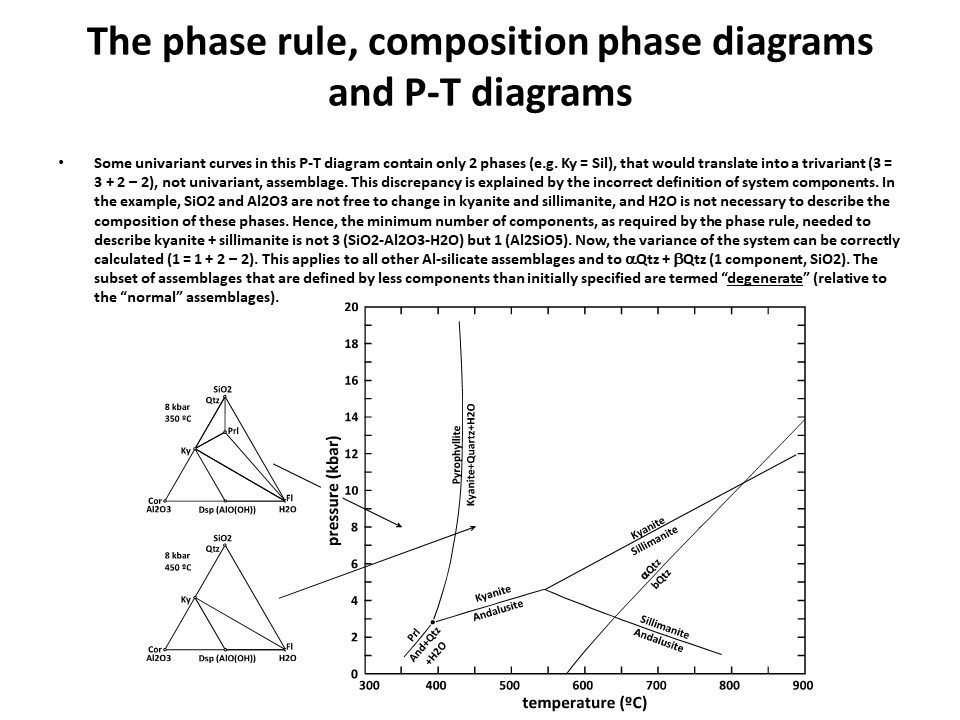
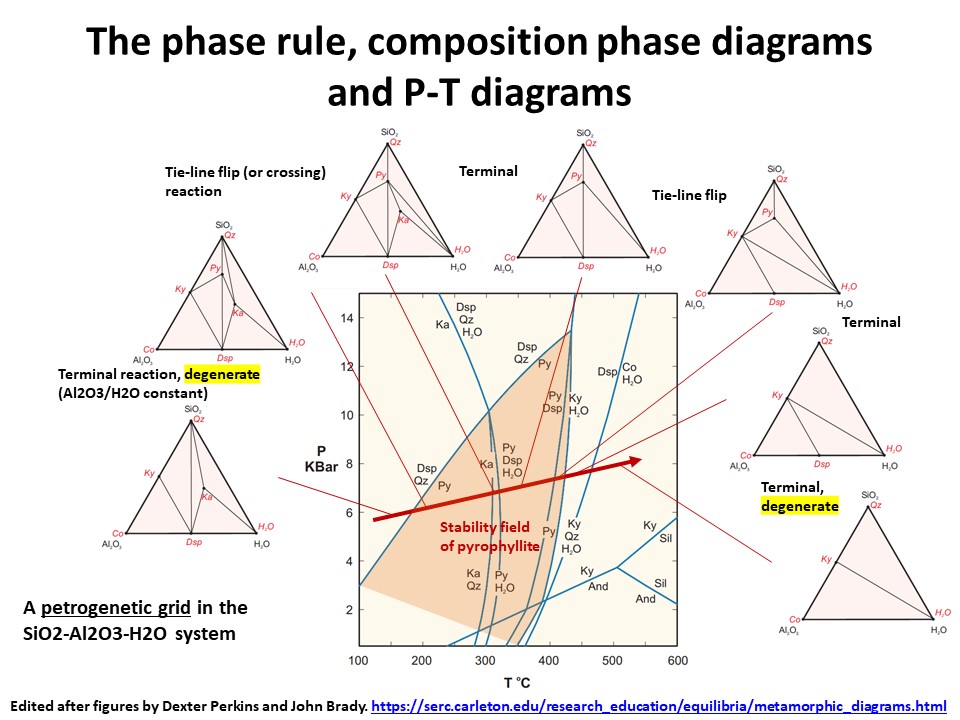
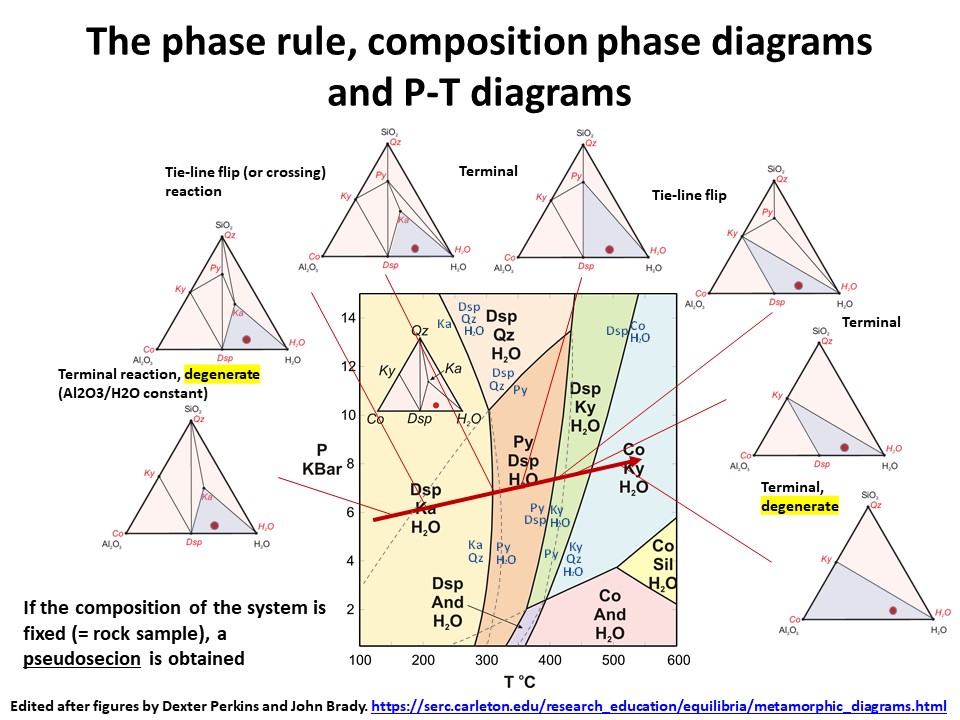
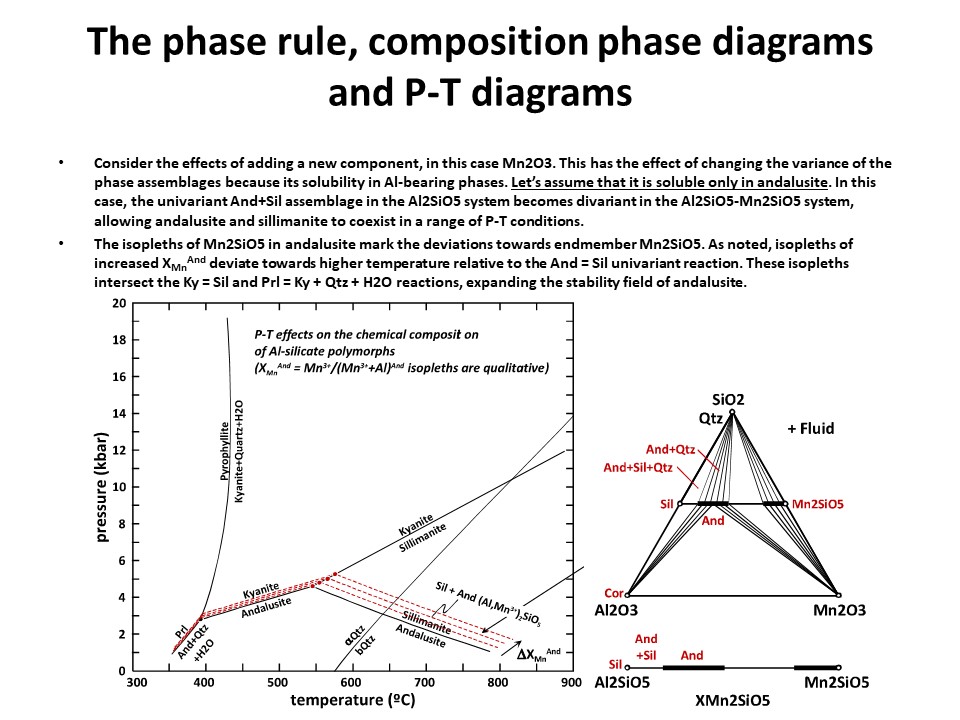
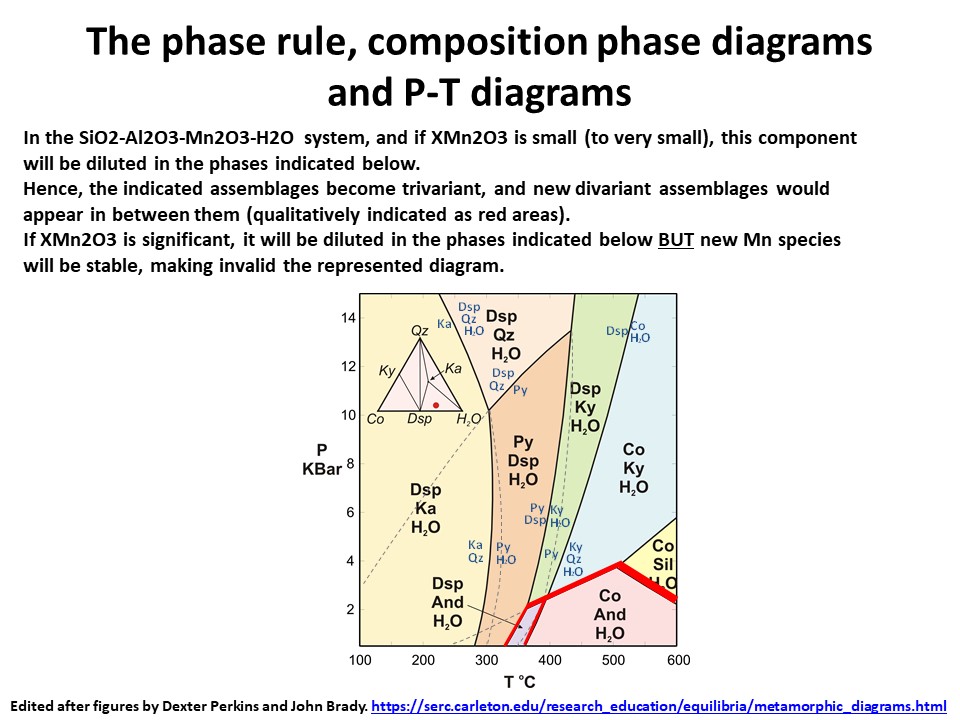
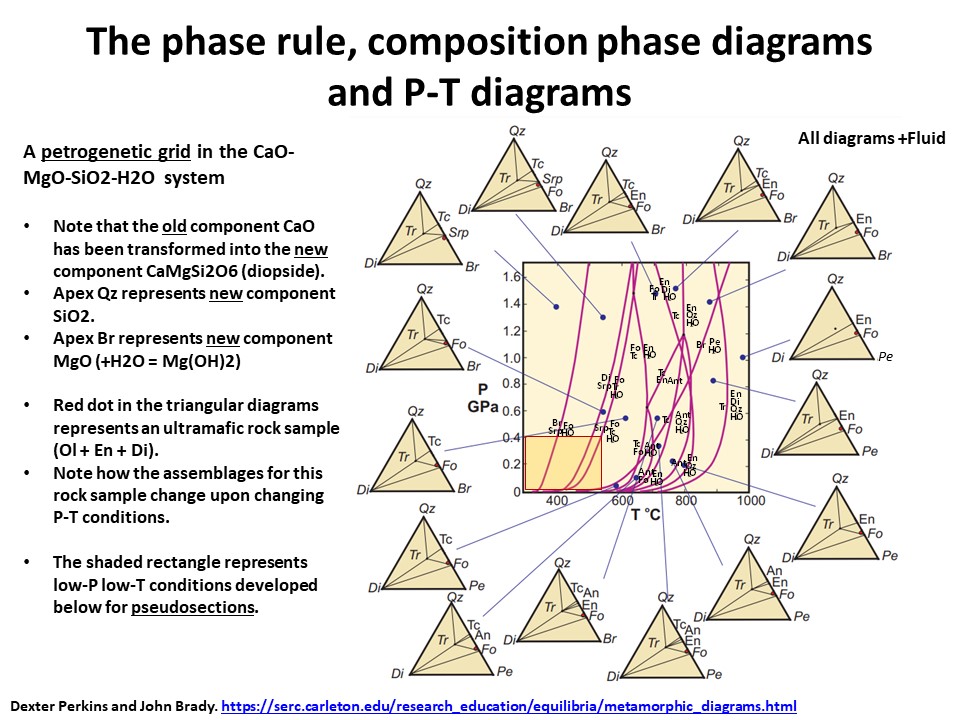
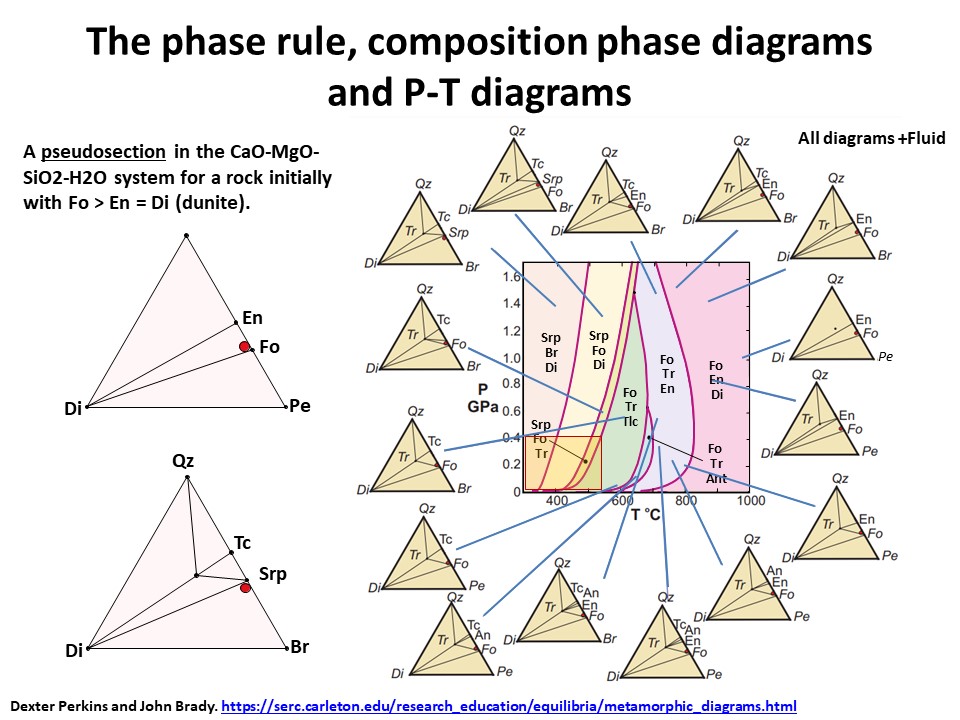
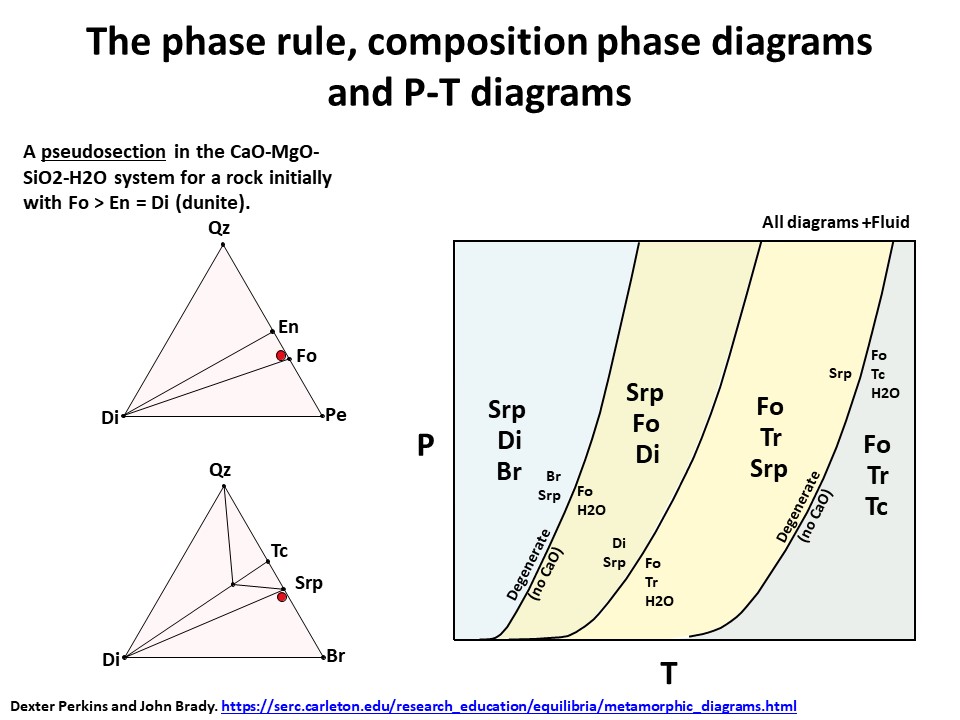
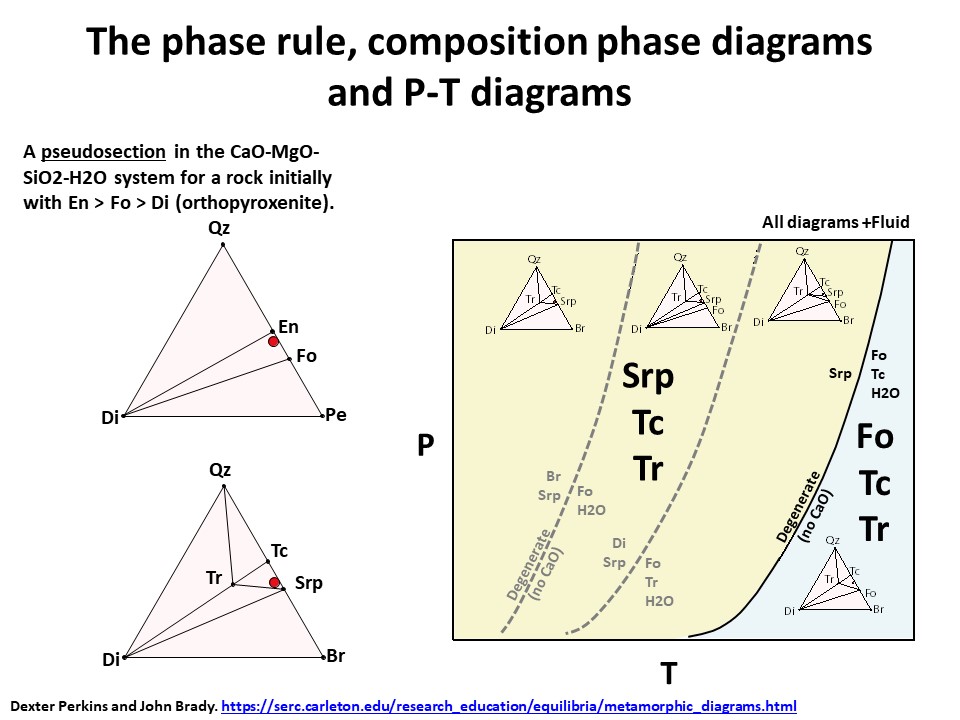
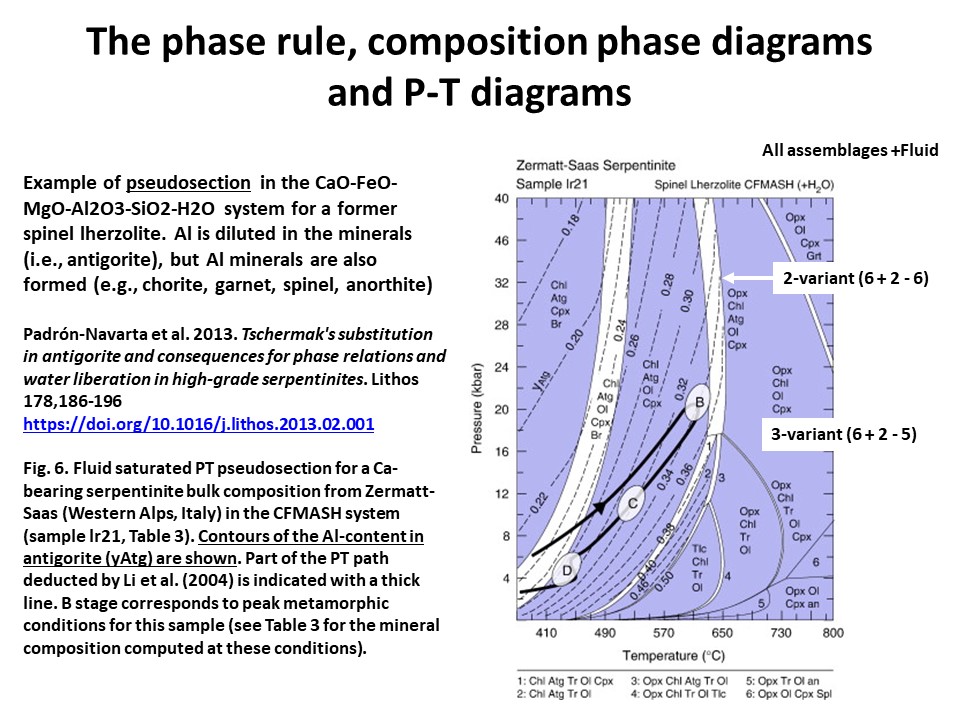
The phase rule, composition phase diagrams and
P-T diagrams
* Number of components + 2 - number of coexisting phases (phase
assemblage) equals the thermodynamic variance (or degrees of
freedom) of the phase assemblage.
|