|
Structural (geometries) and spectroscopic (NMR chemical shifts) characterization has been performed
for a series of modified a- and b-cyclodextrins (CD),
using the density functional theory (B3LYP/6-31G*)
in combination with the GIAO method for calculating of the 13C and 1H chemical shifts.
The compounds studied corresponded to the functionalized primary hydroxy group at C-6 (R = H, F, Cl)
for all the sugar moieties (per-substituted CDs) in the a- and b-CDs.
Comparisons between experimental
and calculated dH and dC chemical shifts yielded
a good agreement for the DFT geometries. Furthermore,
the GIAO-DFT methods proved to be a reliable tool in the assignments of the experimental NMR spectra.
Finally, the 3JH,H vicinal coupling constants were also estimated according with the
Haasnoot-Leeuw-Altona equation and the results were compared with experimental data.
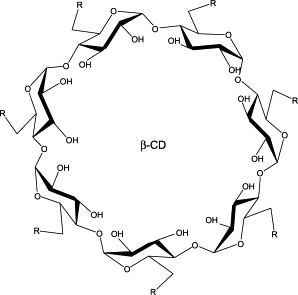 General structure for a per-6-substituted β-CD.
|



