|
The isomerization of β-caryophyllene (3), under treatment with SeO2, is described. Chemical correlations, between 3 and 14-hydroxy-β-caryophyllene (6) from Juniperus oxycedrus, are established. High resolution H-1 NMR spectra and analysis by molecular mechanics (MM) of 3, 6 and 14-acetoxy-β-taryophyllene (1) indicate the existence of two conformational isomers, βα and ββ, in each compound. At 25 degrees C, the βα conformer predominates in 3 and 7 but the ββ conformer predominates in 6. The higher percentage of 6 ββ possibly derives from an intramolecular hydrogen bond. The treatment of 3, 6 and 7 with m-CPBA generates, in each case, two diastereomeric 4,5-epoxi-derivatives. The epoxides obtained from 6 have been isolated and analysed separately.
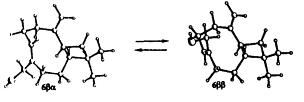
|



