Research Interest
- Low temperature catalytic elimination of VOC
- Development of advanced carbon materials
- Removing pollutants from water by catalytic oxidation processes
- Selective catalytic hydrogenations
- CO2 electro-catalytic reduction to hydrocarbon
Publications, Citations and Researcher ID's
Google Scholar
https://scholar.google.es/citations?hl=es&user=x4cwF2UAAAAJ
ORCID ID: 0000-0002-1483-003
http://orcid.org/0000-0002-1483-0035
Web of Science ResearcherID: F-1243-2010
https://publons.com/researcher/2831556/agustin-f-perez-cadenas/
ResearchGate
https://www.researchgate.net/profile/Agustin_Perez-Cadenas
Directory UGR
https://directorio.ugr.es/static/PersonalUGR/*/show/c04eb985feac2575ea75e1ce419fc802
Membership of Professional Societies:
- The Spanish Carbon Group (GEC), which is integrated in the European Carbon Association (ECA).
Low temperature catalytic elimination of VOC

Volatile organic compounds (VOC) are major air pollutants, and catalytic combustion is one of the most important technologies for eliminating VOC present at low concentration in effluent streams. From an energetic point of view, and to avoid NOx formation, it is very important for this combustion to take place at low temperatures (below 200 ºC), however, in these conditions, the water molecules produced or present in ambient air, can be retained on the catalytic support with negative effects (inhibition) on the activity of the catalyst.
For this reason, the hydrophobicity of carbon materials has led to recent proposals for their use as support for catalysts in the total VOC combustion. Thus, the known advantages in catalysis of the ceramic monolithic-supports (low pressure drops, short diffusion distances and the lack of attrition by vibrational and thermal shock resistance) and of carbon materials (versatility in surface area, pore texture and surface chemistry) make a good combination for VOC applied catalytic combustion.
[go back]
Development of advanced carbon materials

Carbon aerogels, xerogels, nanofibers... carbon composites.
Development of tailored micro-, meso- or macroporous carbon materials, doped-carbon materials, graphitic materials, as well as carbon-based catalysts with very high dispersion and cheaper and shorter synthesis processes. Advanced carbon materials are also obtained as coating of ceramic structures.
[go back]
Removing pollutants from water by advanced oxidation processes

The catalytic performance of metal-supported and metal-doped carbon aerogels and xerogeles has been studied in the elimination of different pollutants in aqueous solutions by CWAO and Fenton like processes.
[go back]
Catalytic selective hydrogenations
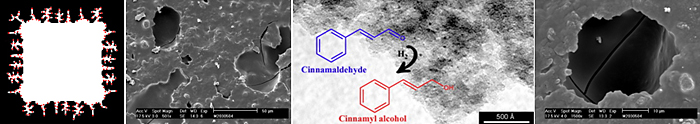
The type of porosity of carbon materials can strongly affect the activity and selectivity in selective hydrogenations if used as catalyst support. Carbon-coated monoliths have shown very good performance in the Pd catalyzed selective hydrogenation of fatty acid methyl esters (FAMEs).
Porosity and chemical surface characteristics of carbon materials are crucial when they are used as catalyst supports in the selective hydrogenation of α,β-unsaturated compounds.
[go back]
CO2 electro-catalytic reduction to hydrocarbons
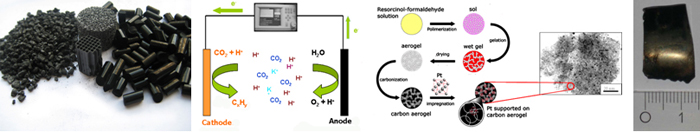
CO2 is a well-known greenhouse gas, and its increasing atmospheric concentration is thought to be one of the main causes of global climate change. In particular, CO2 emission from the use of fossil fuels contributes to the increasing concentration, because it constitutes a continuous net increase in the natural cycle of tropospheric carbon. Renewable energy sources thus receive a lot of attention. However, many renewable energy sources such as solar, wind and tidal electricity do not produce the constant and tunable currents that fossil fuels provide. Storage of surplus electrical energy produced during peak production periods and its release during peak demand periods is thus crucial, especially as peak production and peak demand periods often do not coincide.
An new strategy to address the problem of storing temporary and local surpluses of renewable energy is the electro-catalytic reduction of CO2 to hydrocarbons. In a one-pot process, water is split to provide the required hydrogen atoms / ions which are reacted with CO2 to form hydrocarbons that can be used directly in the existing infrastructure for transportation fuels. Next to storing the renewable energy, some CO2 is removed from the atmosphere. O2 is formed simultaneously, which can be used to burn the hydrocarbons to release the energy whenever required. We have demonstrated the application of metal-doped carbon gels as promising electrodes and electro-catalysts in the electro-catalytic reduction of CO2 to hydrocarbons, whose first results have been recently patented.
[go back]
